Hydrogen ionic conductors and ammonia conversions
IF 3.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 1
Abstract
Electrochemical and catalytic conversion to and from ammonia is strongly enhanced by appropriate choice of hydrogen conducting electrolyte or substrate. Here we explore both protonic and hydride ionic conductors in relation to ammonia conversions. Protonic conductors tend to require too high a temperature to achieve sufficient hydrogen flux for ammonia synthesis as thermal decomposition competes strongly. Conversely protonic conductors are well suited to direct ammonia fuel cell use. Hydride ions can be very mobile and are strongly reducing. Alkaline hydride lattices can exhibit facile H and N mobility and exchange and offer a very promising basis for ammonia conversion and synthesis.
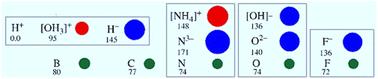
氢离子导体和氨转化
适当选择导氢电解质或衬底,可大大提高氨的电化学和催化转化。在这里,我们探讨质子和氢化物离子导体与氨转化的关系。由于热分解的激烈竞争,质子导体往往需要过高的温度来获得足够的氢通量来合成氨。相反,质子导体非常适合直接用于氨燃料电池。氢化物离子具有很强的流动性和还原性。碱性氢化物晶格具有较好的H、N迁移和交换能力,为氨转化和合成提供了良好的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Faraday Discussions
化学-物理化学
自引率
0.00%
发文量
259
期刊介绍:
Discussion summary and research papers from discussion meetings that focus on rapidly developing areas of physical chemistry and its interfaces
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


