Synthesis of 2,4-diarylated pyrimidines enabled by Ni-catalyzed C–sulfone bond activation†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d2qo01935c
引用次数: 0
Abstract
Herein, we report the use of the readily available and bench-stable pyrimidinyl sulfones as electrophiles in Ni-catalyzed Suzuki−Miyaura cross-coupling reactions. This method allows the facile synthesis of 2,4-disubstituted pyrimidines that are pharmaceutically relevant. These reactions were carried out under mild conditions and show a remarkable substrate scope. In this approach, the C–sulfone bond is cleaved efficiently and regioselectively. Preliminary mechanistic studies revealed the importance of the α-nitrogen atom in pyrimidine in facilitating these transformations.
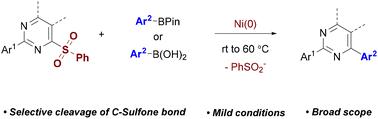
镍催化c -砜键活化合成2,4-二芳化嘧啶
在此,我们报道了在镍催化的Suzuki−Miyaura交叉偶联反应中使用易得且台架稳定的嘧啶基砜作为亲电试剂。该方法允许容易地合成药学上相关的2,4-二取代嘧啶。这些反应在温和的条件下进行,并显示出显著的底物范围。在这种方法中,C–砜键被有效且区域选择性地裂解。初步的机制研究揭示了嘧啶中α-氮原子在促进这些转化中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


