The α-alkylation of carbonyl sulfoxonium ylides: studies and applications in the synthesis of new sulfur heterocycles†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00688c
引用次数: 1
Abstract
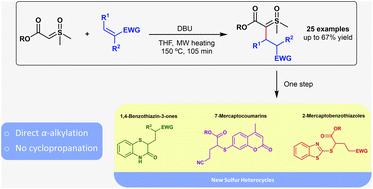
The challenging direct α-alkylation of sulfoxonium ylides is demonstrated after a deep study with several electrophiles. Twenty-five alkylated ester sulfoxonium ylides could be prepared in 12–67% isolated yields, employing Michael acceptors as electrophiles. Interestingly, no product arising from the classical cyclopropanation reaction, commonly observed for the reaction between sulfur ylides and Michael acceptors, was observed. To demonstrate the applicability of these unprecedented and more decorated sulfoxonium ylides, new and medicinal-chemistry important sulfur heterocycles, such as 2-mercaptobenzothiazoles, 1,4-benzothiazin-3-ones, and coumarin derivatives, were prepared.
羰基亚砜鎓化物的α-烷基化:在新型硫杂环合成中的研究与应用
在对几种亲电试剂进行深入研究后,证明了磺酰亚砜的直接α-烷基化具有挑战性。使用迈克尔受体作为亲电试剂,可以以12-67%的分离产率制备25种烷基化酯磺基亚砜。有趣的是,没有观察到来自经典环丙烷化反应的产物,该反应通常在硫叶立德和迈克尔受体之间的反应中观察到。为了证明这些前所未有的、更具修饰性的磺基xonium叶立德的适用性,制备了新的和药物化学重要的硫杂环,如2-巯基苯并噻唑、1,4-苯并噻嗪-3-酮和香豆素衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


