Ahmet Imrali, Christine S. Hughes, Abigail S. Coetzee, Francesca R. Delvecchio, Amina Saad, Rhiannon Roberts, Claude Chelala, Jo-Anne ChinAleong, Hemant M. Kocher
下载PDF
{"title":"Validation of a Novel, Flash-Freezing Method: Aluminum Platform","authors":"Ahmet Imrali, Christine S. Hughes, Abigail S. Coetzee, Francesca R. Delvecchio, Amina Saad, Rhiannon Roberts, Claude Chelala, Jo-Anne ChinAleong, Hemant M. Kocher","doi":"10.1002/cpet.46","DOIUrl":null,"url":null,"abstract":"<p>Stored biological materials should have minimal pre-analytical variations in order to provide researchers with high-quality samples that will give reliable and reproducible results, yet methods of storage should be easy to implement, with minimal cost and health hazard. Frozen tissue samples are a valuable biological resource. Here we compare different methods, such as liquid nitrogen (LN) or dry ice (DI), to a cheap and safe alternative using an aluminum platform (AP). Murine fresh liver and pancreas tissues were used with varying lengths of warm ischemia time. Quality assessment was based on histological evaluation, DNA and RNA extraction and quantification, and RNA degradation analysis, as well preservation of antigens for immunofluorescence, in a blinded manner. Both in superficial and deep tissue sections, based on histological assessment, AP is superior to DI, or as good as LN techniques in terms of presence of ice crystals, cutting artifacts, and overall quality/structural preservation. DNA and RNA were successfully extracted in reasonable quantities from all freezing techniques, but RNA degradation was seen for pancreas samples across all techniques. Immunofluorescence with cytokeratin8 (CK-8), alpha smooth muscle actin (αSMA), CD3, and B220 shows equally good outcomes for AP and LN, which are better than DI. The aluminum platform is a cheap, yet reliable method to freeze samples, rapidly preserving histological, antigenic, and DNA/RNA quality. Wider testing is required across different sample types. © 2020 The Authors.</p><p><b>Basic Protocol</b>: Flash-freezing fresh tissue with aluminum platform</p><p><b>Alternate Protocol 1</b>: Freezing fresh tissue with liquid nitrogen</p><p><b>Alternate Protocol 2</b>: Freezing fresh tissue with dry ice</p>","PeriodicalId":500994,"journal":{"name":"Current Protocols Essential Laboratory Techniques","volume":"21 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-11-25","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpet.46","citationCount":"1","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols Essential Laboratory Techniques","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpet.46","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 1
引用
批量引用
Abstract
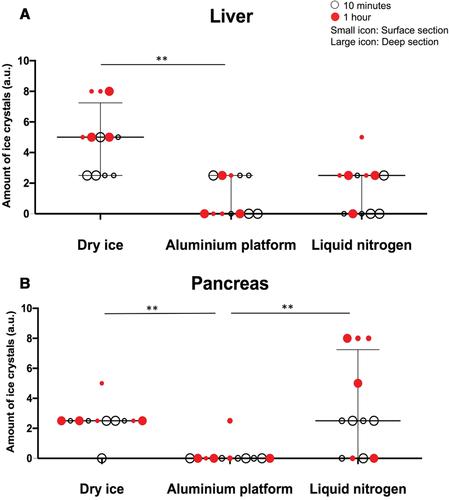
Stored biological materials should have minimal pre-analytical variations in order to provide researchers with high-quality samples that will give reliable and reproducible results, yet methods of storage should be easy to implement, with minimal cost and health hazard. Frozen tissue samples are a valuable biological resource. Here we compare different methods, such as liquid nitrogen (LN) or dry ice (DI), to a cheap and safe alternative using an aluminum platform (AP). Murine fresh liver and pancreas tissues were used with varying lengths of warm ischemia time. Quality assessment was based on histological evaluation, DNA and RNA extraction and quantification, and RNA degradation analysis, as well preservation of antigens for immunofluorescence, in a blinded manner. Both in superficial and deep tissue sections, based on histological assessment, AP is superior to DI, or as good as LN techniques in terms of presence of ice crystals, cutting artifacts, and overall quality/structural preservation. DNA and RNA were successfully extracted in reasonable quantities from all freezing techniques, but RNA degradation was seen for pancreas samples across all techniques. Immunofluorescence with cytokeratin8 (CK-8), alpha smooth muscle actin (αSMA), CD3, and B220 shows equally good outcomes for AP and LN, which are better than DI. The aluminum platform is a cheap, yet reliable method to freeze samples, rapidly preserving histological, antigenic, and DNA/RNA quality. Wider testing is required across different sample types. © 2020 The Authors.
Basic Protocol : Flash-freezing fresh tissue with aluminum platform
Alternate Protocol 1 : Freezing fresh tissue with liquid nitrogen
Alternate Protocol 2 : Freezing fresh tissue with dry ice

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


