Disruption of type I interferon signaling causes sexually dimorphic dysregulation of anti-viral cytokines
Abstract
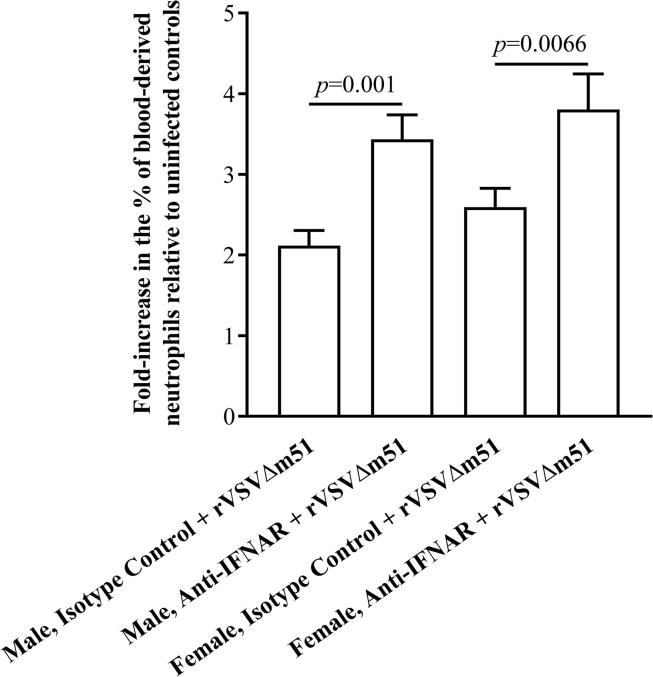
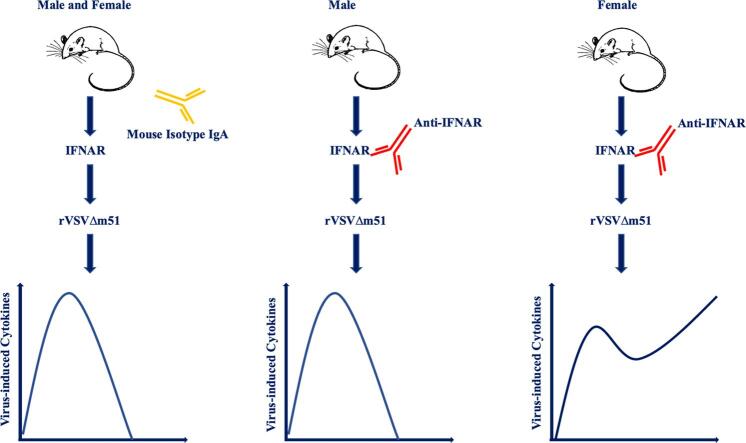
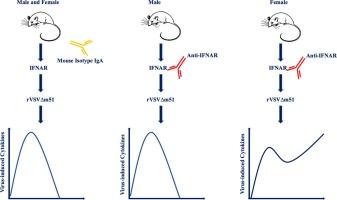
Type I interferons (IFNs) play a crucial role in the establishment of an antiviral state via signaling through their cognate type I IFN receptor (IFNAR). In this study, a replication-competent but highly attenuated strain of VSV (rVSVΔm51) carrying a deletion at position 51 of the matrix protein to remove suppression of anti-viral type I IFN responses was used to explore the effect of disrupted IFNAR signaling on inflammatory cytokine responses in mice. The kinetic responses of interleukin-6, tumor necrosis factor-α and interleukin-12 were evaluated in virus-infected male and female mice with or without concomitant antibody-mediated IFNAR-blockade. Unlike controls, both male and female IFNAR-blocked mice showed signs of sickness by 24-hours post-infection. Female IFNAR-blocked mice experienced greater morbidity as demonstrated by a significant decrease in body temperature. This was not the case for males. In addition, females with IFNAR-blockade mounted prolonged and exaggerated systemic inflammatory cytokine responses to rVSVΔm51. This was in stark contrast to controls with intact IFNAR signaling and males with IFNAR-blockade; they were able to down-regulate virus-induced inflammatory cytokine responses by 24-hours post-infection. Exaggerated cytokine responses in females with impaired IFNAR signaling was associated with more effective control of viremia than their male counterparts. However, the trade-off was greater immune-mediated morbidity. The results of this study demonstrated a role for IFNAR signaling in the down-regulation of antiviral cytokine responses, which was strongly influenced by sex. Our findings suggested that the potential to mount toxic cytokine responses to a virus with concomitant disruption of IFNAR signaling was heavily biased towards females.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


