A chimeric antigen receptor with antigen-independent OX40 signaling mediates potent antitumor activity
IF 15.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 35
Abstract
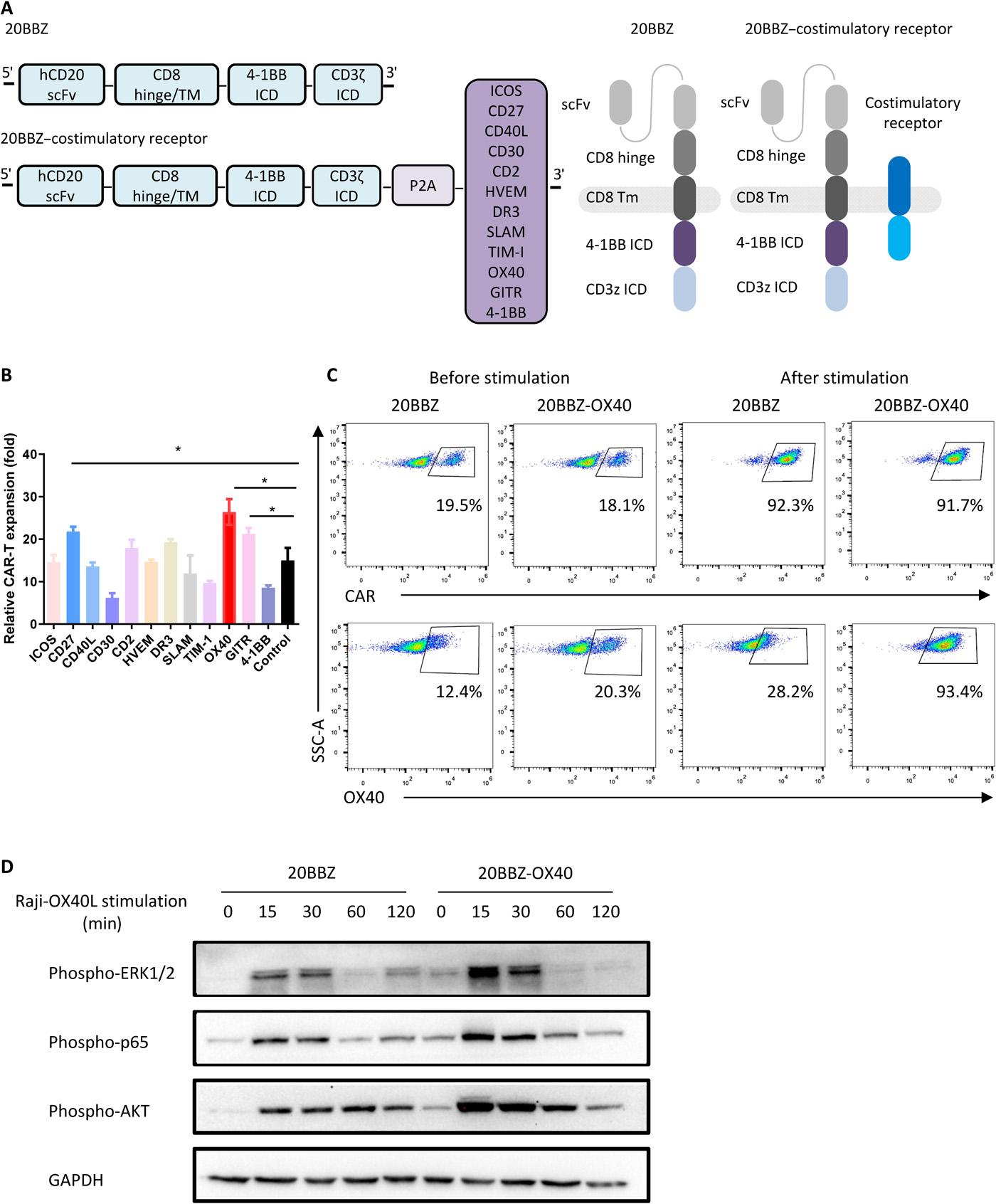
Although chimeric antigen receptor (CAR)–modified T cells have shown great success in the treatment of B cell malignancies, this approach has limited efficacy in patients with solid tumors. Various modifications in CAR structure have been explored to improve this efficacy, including the incorporation of two costimulatory domains. Because costimulatory signals are transduced together with T cell receptor signals during T cell activation, we engineered a type of CAR-T cells with a costimulatory signal that was activated independently from the tumor antigen to recapitulate physiological stimulation. We screened 12 costimulatory receptors to identify OX40 as the most effective CAR-T function enhancer. Our data indicated that these new CAR-T cells showed superior proliferation capability compared to current second-generation CAR-T cells. OX40 signaling reduced CAR-T cell apoptosis through up-regulation of genes encoding Bcl-2 family members and enhanced proliferation through increased activation of the NF-κB (nuclear factor κB), MAPK (mitogen-activated protein kinase), and PI3K-AKT (phosphoinositide 3-kinase to the kinase AKT) pathways. OX40 signaling not only enhanced the cytotoxicity of CAR-T cells but also reduced exhaustion markers, thereby maintaining their function in immunosuppressive tumor microenvironments. In mouse tumor models and in patients with metastatic lymphoma, these CAR-T cells exhibited robust amplification and antitumor activity. Our findings provide an alternative option for CAR-T optimization with the potential to overcome the challenge of treating solid tumors.
具有抗原非依赖性 OX40 信号传导功能的嵌合抗原受体具有强大的抗肿瘤活性
虽然嵌合抗原受体(CAR)修饰的 T 细胞在治疗 B 细胞恶性肿瘤方面取得了巨大成功,但这种方法对实体瘤患者的疗效有限。为了提高疗效,人们对 CAR 结构进行了各种改造,包括加入两个成本调控域。由于在 T 细胞活化过程中, costimulatory 信号与 T 细胞受体信号一起转导,因此我们设计了一种带有 costimulatory 信号的 CAR-T 细胞,该信号独立于肿瘤抗原而被激活,以再现生理刺激。我们筛选了 12 种成本调控受体,发现 OX40 是最有效的 CAR-T 功能增强剂。我们的数据表明,与目前的第二代 CAR-T 细胞相比,这些新型 CAR-T 细胞具有更强的增殖能力。OX40信号通过上调编码Bcl-2家族成员的基因减少了CAR-T细胞的凋亡,并通过增加NF-κB(核因子κB)、MAPK(丝裂原活化蛋白激酶)和PI3K-AKT(磷酸肌酸3-激酶到激酶AKT)通路的活化增强了细胞增殖。OX40信号不仅增强了CAR-T细胞的细胞毒性,还减少了衰竭标志物,从而在免疫抑制性肿瘤微环境中保持了其功能。在小鼠肿瘤模型和转移性淋巴瘤患者中,这些 CAR-T 细胞表现出强大的扩增和抗肿瘤活性。我们的研究结果为CAR-T优化提供了另一种选择,有望克服治疗实体瘤的挑战。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Translational Medicine
CELL BIOLOGY-MEDICINE, RESEARCH & EXPERIMENTAL
CiteScore
26.70
自引率
1.20%
发文量
309
审稿时长
1.7 months
期刊介绍:
Science Translational Medicine is an online journal that focuses on publishing research at the intersection of science, engineering, and medicine. The goal of the journal is to promote human health by providing a platform for researchers from various disciplines to communicate their latest advancements in biomedical, translational, and clinical research.
The journal aims to address the slow translation of scientific knowledge into effective treatments and health measures. It publishes articles that fill the knowledge gaps between preclinical research and medical applications, with a focus on accelerating the translation of knowledge into new ways of preventing, diagnosing, and treating human diseases.
The scope of Science Translational Medicine includes various areas such as cardiovascular disease, immunology/vaccines, metabolism/diabetes/obesity, neuroscience/neurology/psychiatry, cancer, infectious diseases, policy, behavior, bioengineering, chemical genomics/drug discovery, imaging, applied physical sciences, medical nanotechnology, drug delivery, biomarkers, gene therapy/regenerative medicine, toxicology and pharmacokinetics, data mining, cell culture, animal and human studies, medical informatics, and other interdisciplinary approaches to medicine.
The target audience of the journal includes researchers and management in academia, government, and the biotechnology and pharmaceutical industries. It is also relevant to physician scientists, regulators, policy makers, investors, business developers, and funding agencies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


