Real-World Outcomes of Treating Advanced Breast Cancer Patients With Palbociclib: A Multicenter Retrospective Cohort Study in Japan-The KBCOG-14 Study.
IF 1.8
Q3 ONCOLOGY
Breast Cancer : Basic and Clinical Research
Pub Date : 2020-12-28
eCollection Date: 2020-01-01
DOI:10.1177/1178223420983843
引用次数: 10
Abstract
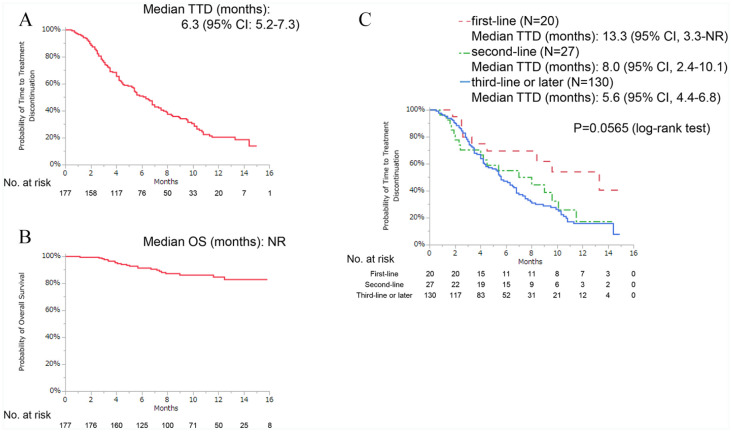
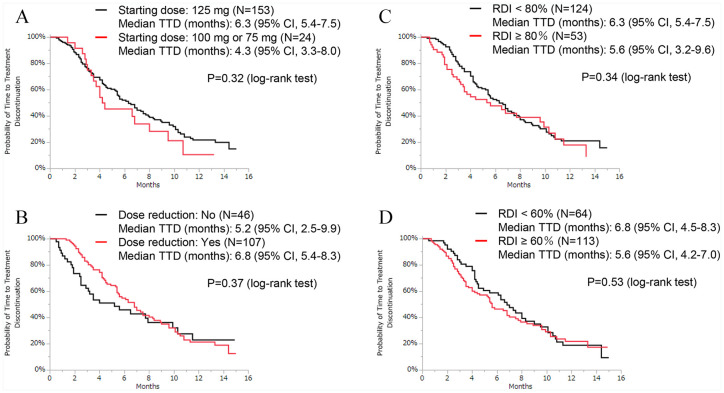
Background: Clinical studies have shown that palbociclib improves progression-free survival in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2−) patients with advanced breast cancer (ABC). However, there are insufficient data on its use in a real-world setting in Japan. The aim of this study was to investigate the effectiveness, predictive factors, and safety of palbociclib among Japanese patients in routine clinical practice. Methods: Between December 1, 2017, and April 30, 2019, we recruited patients from 9 hospitals and retrospectively evaluated the data on HR+/HER2− patients with ABC who received palbociclib for at least 1 week. The correlation between time-to-treatment discontinuation (TTD) and clinical background was investigated via univariate and multivariate analyses using Cox hazards models. Results: A total of 177 women were available for analysis. Of these patients, 58 (33%) patients were treated with palbociclib with an aromatase inhibitor and 117 (66%) patients were treated with palbociclib and a selective estrogen receptor degrader. Approximately three-fourths of the patients (n = 130, 73%) received palbociclib as third- or later-line therapy. One-third of the patients had 3 or more metastatic sites (n = 59, 33%), and one-third of the patients had liver metastasis (n = 59, 33%). The median follow-up duration at the time of data cutoff was 8.9 months, the median TTD was 6.3 months, and the median overall survival was not reached. Liver metastasis (hazard ratio [HR]: 1.54 [95% confidence interval {CI}: 1.03-2.27]), high serum lactate dehydrogenase (LDH) level (>300 U/L) (HR: 2.58 [95% CI: 1.49-4.26]), and high neutrophil-to-lymphocyte ratio (NLR) (⩾3.0) (HR: 1.76 [95% CI: 1.13-2.69]) were significantly associated with shorter TTD. The most common hematologic adverse event was neutropenia, which occurred in 93% of the patients. Conclusion: Based on the results of the pivotal phase 3 trials, the median TTD recorded in this study was shorter than expected. Our results suggest that liver metastasis, serum LDH level, and NLR may be predictive factors for HR+/HER2− ABC treatment outcomes.

帕博西尼治疗晚期乳腺癌患者的实际结果:日本的一项多中心回顾性队列研究——KBCOG-14研究
背景:临床研究表明,帕博西尼可改善激素受体阳性(HR+)、人表皮生长因子受体2阴性(HER2-)晚期乳腺癌(ABC)患者的无进展生存期。然而,关于它在日本现实环境中的使用数据不足。本研究的目的是调查帕博西尼在日本患者常规临床实践中的有效性、预测因素和安全性。方法:在2017年12月1日至2019年4月30日期间,我们从9家医院招募患者,回顾性评估接受帕博西尼治疗至少1周的HR+/HER2- ABC患者的数据。采用Cox风险模型进行单因素和多因素分析,研究停药时间(TTD)与临床背景的相关性。结果:共有177名妇女可用于分析。在这些患者中,58例(33%)患者使用帕博西尼联合芳香化酶抑制剂治疗,117例(66%)患者使用帕博西尼联合选择性雌激素受体降解剂治疗。大约四分之三的患者(n = 130,73%)接受帕博西尼作为三线或后期治疗。三分之一的患者有3个及以上转移部位(n = 59, 33%),三分之一的患者有肝转移(n = 59, 33%)。数据截止时的中位随访时间为8.9个月,中位TTD为6.3个月,中位总生存期未达到。肝转移(风险比[HR]: 1.54[95%可信区间{CI}: 1.03-2.27])、高血清乳酸脱氢酶(LDH)水平(>300 U/L) (HR: 2.58 [95% CI: 1.49-4.26])和高中性粒细胞与淋巴细胞比率(NLR) (HR: 1.76 [95% CI: 1.13-2.69])与较短的TTD显著相关。最常见的血液学不良事件是中性粒细胞减少,发生率为93%。结论:基于关键3期试验的结果,本研究记录的中位TTD比预期短。我们的研究结果表明,肝转移、血清LDH水平和NLR可能是HR+/HER2- ABC治疗结果的预测因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.10
自引率
3.40%
发文量
22
审稿时长
8 weeks
期刊介绍:
Breast Cancer: Basic and Clinical Research is an international, open access, peer-reviewed, journal which considers manuscripts on all areas of breast cancer research and treatment. We welcome original research, short notes, case studies and review articles related to breast cancer-related research. Specific areas of interest include, but are not limited to, breast cancer sub types, pathobiology, metastasis, genetics and epigenetics, mammary gland biology, breast cancer models, prevention, detection, therapy and clinical interventions, and epidemiology and population genetics.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


