Low Dose of β-Carotene Regulates Inflammation, Reduces Caspase Signaling, and Correlates with Autophagy Activation in Cardiomyoblast Cell Lines.
IF 2
Q3 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 7
Abstract
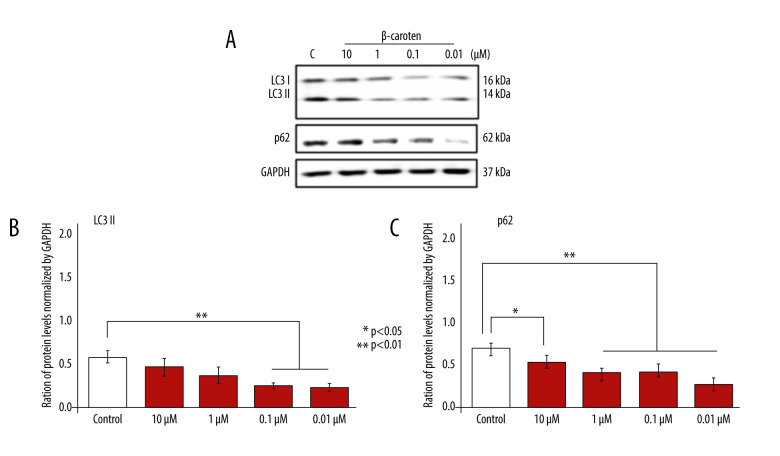
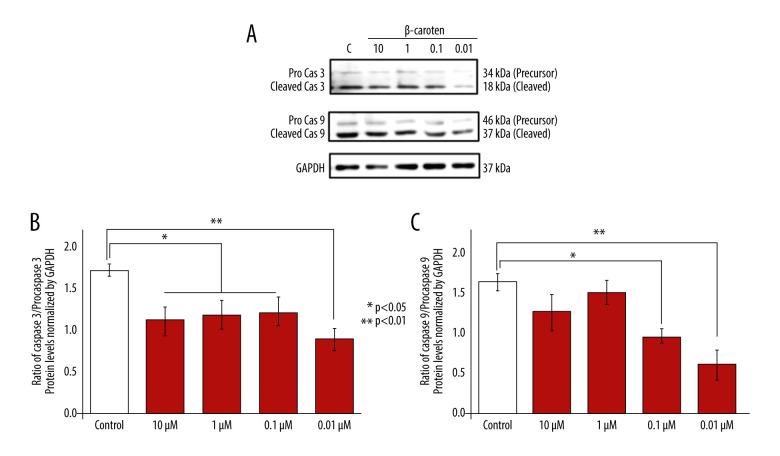
Background Excessive reactive oxygen species (ROS) stimulate mitochondrial damage that causes degenerative diseases such as cardiovascular disease (CVD). β-carotene (BC), a natural antioxidant able to counteract free radicals, acts as a cytoprotective agent. However, knowledge of the role of BC on cardiomyoblasts is limited. In this study, we explored its role on COX4, Tom20, Nfr1, Nrf2, Nf-κB, LC3, p62, caspase 3, and caspase 9 and its association with cardiomyoblast viability and survival. Material/Methods H9C2 cell lines were seeded, cultivated until 90% to 100% confluency, and treated with various doses of BC: 10 μM, 1 μM, 0.1 μM, and 0.01 μM. After 24 h, the cells were harvested, lyzed, and tested for specific related protein expressions from each dose. Results Low-dose BC induced autophagy most effectively at 1 μM, 0.1 μM, and 0.01 μM, as indicated by a decrease of LC3II and p62 levels. We observed that Nf-κB protein levels were suppressed; Nrf2 was stimulated, but Nrf1 was not altered significantly. Further, low-dose BC might stimulate cell viability by reducing apoptotic signals of caspase 3 and 9. Notably, low-dose BC also showed potential to increase Tom20 protein levels. Conclusions Low-dose BC supplementation shows beneficial effects, especially at 0.01 μM, by reducing inflammation through the suppression of Nf-κB and increase of Nrf2 level. Autophagy as a cellular maintenance mechanism was also stimulated, and the amount of the mitochondria marker Tom20 increased. Taken together, results showed that specific low-dose BC is effective and might improve cell viability by stimulating autophagy, inhibiting proinflammatory factors, and suppressing apoptosis.
低剂量β-胡萝卜素调节成心肌细胞炎症、减少Caspase信号传导并与自噬激活相关
过多的活性氧(ROS)刺激线粒体损伤,导致退行性疾病,如心血管疾病(CVD)。ß-胡萝卜素(BC)是一种天然抗氧化剂,能够抵抗自由基,起到细胞保护剂的作用。然而,关于BC对心肌细胞的作用的知识是有限的。在这项研究中,我们探讨了它在COX4、Tom20、Nfr1、Nrf2、Nf-kappaB、LC3、p62、caspase 3和caspase 9中的作用及其与成心肌细胞活力和生存的关系。材料和方法将H9C2细胞系播种,培养至90%至100%的融合度,并用不同剂量的BC处理:10µM, 1µM, 0.1µM和0.01µM。24小时后,收获细胞,分析细胞,并检测每次剂量的特异性相关蛋白表达。结果低剂量BC在1µM、0.1µM和0.01µM时诱导自噬最有效,LC3II和p62水平降低。我们观察到Nf-kB蛋白水平被抑制;Nrf2受到刺激,但Nrf1无明显改变。此外,低剂量的BC可能通过减少caspase 3和9的凋亡信号来刺激细胞活力。值得注意的是,低剂量的BC也显示出增加Tom20蛋白水平的潜力。结论低剂量BC可通过抑制Nf-kappaB和增加Nrf2水平来减轻炎症,特别是在0.01µM时。自噬作为一种细胞维持机制也受到刺激,线粒体标记物Tom20的数量增加。综上所述,结果表明,特定低剂量的BC是有效的,可能通过刺激自噬、抑制促炎因子和抑制细胞凋亡来提高细胞活力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Medical Science Monitor Basic Research
MEDICINE, RESEARCH & EXPERIMENTAL-
CiteScore
6.00
自引率
0.00%
发文量
16
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


