Programmable Gene Knockdown in Diverse Bacteria Using Mobile-CRISPRi
Amy B. Banta, Ryan D. Ward, Jennifer S. Tran, Emily E. Bacon, Jason M. Peters
下载PDF
{"title":"Programmable Gene Knockdown in Diverse Bacteria Using Mobile-CRISPRi","authors":"Amy B. Banta, Ryan D. Ward, Jennifer S. Tran, Emily E. Bacon, Jason M. Peters","doi":"10.1002/cpmc.130","DOIUrl":null,"url":null,"abstract":"<p>Facile bacterial genome sequencing has unlocked a veritable treasure trove of novel genes awaiting functional exploration. To make the most of this opportunity requires powerful genetic tools that can target all genes in diverse bacteria. CRISPR interference (CRISPRi) is a programmable gene-knockdown tool that uses an RNA-protein complex comprised of a single guide RNA (sgRNA) and a catalytically inactive Cas9 nuclease (dCas9) to sterically block transcription of target genes. We previously developed a suite of modular CRISPRi systems that transfer by conjugation and integrate into the genomes of diverse bacteria, which we call Mobile-CRISPRi. Here, we provide detailed protocols for the modification and transfer of Mobile-CRISPRi vectors for the purpose of knocking down target genes in bacteria of interest. We further discuss strategies for optimizing Mobile-CRISPRi knockdown, transfer, and integration. We cover the following basic protocols: sgRNA design, cloning new sgRNA spacers into Mobile-CRISPRi vectors, Tn<i>7</i> transfer of Mobile-CRISPRi to Gram-negative bacteria, and ICE<i>Bs1</i> transfer of Mobile-CRISPRi to Bacillales. © 2020 The Authors.</p><p><b>Basic Protocol 1</b>: sgRNA design</p><p><b>Basic Protocol 2</b>: Cloning of new sgRNA spacers into Mobile-CRISPRi vectors</p><p><b>Basic Protocol 3</b>: Tn<i>7</i> transfer of Mobile-CRISPRi to Gram-negative bacteria</p><p><b>Basic Protocol 4</b>: ICE<i>Bs1</i> transfer of Mobile-CRISPRi to Bacillales</p><p><b>Support Protocol 1</b>: Quantification of CRISPRi repression using fluorescent reporters</p><p><b>Support Protocol 2</b>: Testing for gene essentiality using CRISPRi spot assays on plates</p><p><b>Support Protocol 3</b>: Transformation of <i>E. coli</i> by electroporation</p><p><b>Support Protocol 4</b>: Transformation of CaCl<sub>2</sub>-competent <i>E. coli</i></p>","PeriodicalId":39967,"journal":{"name":"Current Protocols in Microbiology","volume":"59 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-12-17","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpmc.130","citationCount":"7","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols in Microbiology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpmc.130","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 7
引用
批量引用
Abstract
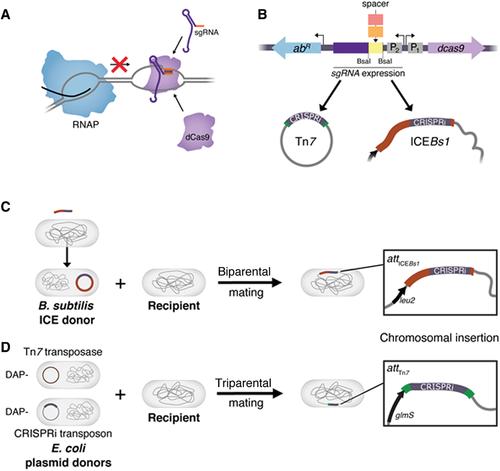
Facile bacterial genome sequencing has unlocked a veritable treasure trove of novel genes awaiting functional exploration. To make the most of this opportunity requires powerful genetic tools that can target all genes in diverse bacteria. CRISPR interference (CRISPRi) is a programmable gene-knockdown tool that uses an RNA-protein complex comprised of a single guide RNA (sgRNA) and a catalytically inactive Cas9 nuclease (dCas9) to sterically block transcription of target genes. We previously developed a suite of modular CRISPRi systems that transfer by conjugation and integrate into the genomes of diverse bacteria, which we call Mobile-CRISPRi. Here, we provide detailed protocols for the modification and transfer of Mobile-CRISPRi vectors for the purpose of knocking down target genes in bacteria of interest. We further discuss strategies for optimizing Mobile-CRISPRi knockdown, transfer, and integration. We cover the following basic protocols: sgRNA design, cloning new sgRNA spacers into Mobile-CRISPRi vectors, Tn7 transfer of Mobile-CRISPRi to Gram-negative bacteria, and ICEBs1 transfer of Mobile-CRISPRi to Bacillales. © 2020 The Authors.
Basic Protocol 1 : sgRNA design
Basic Protocol 2 : Cloning of new sgRNA spacers into Mobile-CRISPRi vectors
Basic Protocol 3 : Tn7 transfer of Mobile-CRISPRi to Gram-negative bacteria
Basic Protocol 4 : ICEBs1 transfer of Mobile-CRISPRi to Bacillales
Support Protocol 1 : Quantification of CRISPRi repression using fluorescent reporters
Support Protocol 2 : Testing for gene essentiality using CRISPRi spot assays on plates
Support Protocol 3 : Transformation of E. coli by electroporation
Support Protocol 4 : Transformation of CaCl2 -competent E. coli
使用Mobile-CRISPRi在多种细菌中可编程基因敲除
简单的细菌基因组测序已经打开了等待功能探索的新基因的真正宝库。为了充分利用这一机会,需要强大的基因工具,可以针对不同细菌中的所有基因。CRISPR干扰(CRISPRi)是一种可编程的基因敲低工具,它使用由单个引导RNA (sgRNA)和催化失活的Cas9核酸酶(dCas9)组成的RNA-蛋白复合物来立体阻断靶基因的转录。我们之前开发了一套模块化的CRISPRi系统,通过接合转移并整合到不同细菌的基因组中,我们称之为Mobile-CRISPRi。在这里,我们为Mobile-CRISPRi载体的修饰和转移提供了详细的协议,目的是敲除感兴趣的细菌中的靶基因。我们进一步讨论了优化Mobile-CRISPRi敲除、转移和整合的策略。我们介绍了以下基本方案:sgRNA设计,将新的sgRNA间隔物克隆到Mobile-CRISPRi载体中,Tn7将Mobile-CRISPRi转移到革兰氏阴性菌中,以及ICEBs1将Mobile-CRISPRi转移到芽胞杆菌中。©2020作者。基本方案1:sgRNA设计基本方案2:将新的sgRNA间隔物克隆到Mobile-CRISPRi载体基本方案3:Tn7将Mobile-CRISPRi转移到革兰氏阴性细菌基本方案4:icicbs1将Mobile-CRISPRi转移到芽孢杆菌支持方案1:使用荧光报告器定量CRISPRi抑制支持方案2:使用CRISPRi在平板上进行斑点分析测试基因必要性支持方案3:通过电孔转化大肠杆菌支持方案4:cacl2态大肠杆菌的转化
本文章由计算机程序翻译,如有差异,请以英文原文为准。
来源期刊
期刊介绍:
Current Protocols in Microbiology provides detailed, step-by-step instructions for analyzing bacteria, animal and plant viruses, fungi, protozoans and other microbes. It offers updated coverage of emerging technologies and concepts, such as biofilms, quorum sensing and quantitative PCR, as well as proteomic and genomic methods. It is the first comprehensive source of high-quality microbiology protocols that reflects and incorporates the new mandates and capabilities of this robust and rapidly evolving discipline.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


