From benzopyrroles to phenylpyrroles: remodeling of indoles enabled by photoredox catalysis†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00505d
引用次数: 0
Abstract
A facile approach toward 3-(o-aminophenyl)pyrroles was developed by remodeling of N-sulfonyl-3-acyl indoles with N-phenylglycines under photoredox catalysis. This strategy enables the conversion of a fused bicyclic system into a biaryl scaffold, mechanically involving a Giese-type radical addition to C2C3, C2–N cleavage of the resulting indoline skeleton, and generation of a new pyrrole motif. This novel methodology provides an innovative strategy for the synthesis of potentially valuable 3-(o-aminophenyl)pyrroles and related alkaloids.
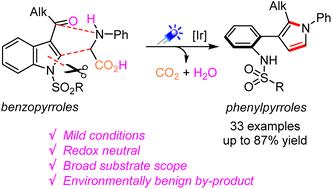
从苯并吡咯到苯基吡咯:光氧化还原催化实现吲哚的重塑†
通过在光氧化还原催化下用N-苯基甘氨酸重构N-磺酰基-3-酰基吲哚,开发了一种制备3-(邻氨基苯基)吡咯的简单方法。该策略能够将稠合双环系统转化为二芳基支架,机械地将Giese型自由基添加到C2C3,C2–N切割得到的吲哚啉骨架,并产生新的吡咯基序。这种新方法为合成潜在价值的3-(邻氨基苯基)吡咯和相关生物碱提供了一种创新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


