Facile access to 1-aryl-2,3-naphthalimides via consecutive amidation/dehydro-Diels–Alder reactions†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00395g
引用次数: 0
Abstract
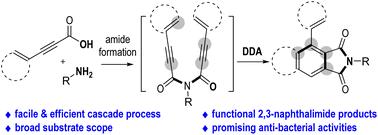
A facile approach for quick and efficient access to structurally complex 2,3-naphthalimide derivatives is developed. Easily accessible and inexpensive β-arylpropiolic acids and primary amines are used as the reaction starting materials. Both reactants can tolerate a diversity of substituents with various electronic and steric effects. Multi-functional 1-aryl-2,3-naphthalimides are afforded in generally moderate to excellent yields through a consecutive cascade amidation/dehydro-Diels–Alder reaction in one-pot operations. Promising applications of the afforded 1-aryl-2,3-naphthalimide products in bactericide development for plant protections are also exhibited.
通过连续的酰胺化/脱氢Diels-Alder反应促进1-芳基-2,3-邻苯二甲酰亚胺的获取†
开发了一种快速有效地获得结构复杂的2,3-邻苯二甲酰亚胺衍生物的简便方法。使用容易获得且廉价的β-芳基丙酸和伯胺作为反应起始材料。两种反应物都能耐受具有各种电子和空间效应的各种取代基。多官能1-芳基-2,3-邻苯二甲酰亚胺通常通过一锅操作中的连续级联酰胺化/脱氢Diels-Alder反应以中等至优异的产率提供。还展示了所提供的1-芳基-2,3-邻苯二甲酰亚胺产品在植物保护杀菌剂开发中的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


