Palladium-catalyzed asymmetric (4 + 3) cycloaddition of N-2,2,2-trifluoroethylisatin ketimines: access to optically active spirooxindoles†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00501a
引用次数: 0
Abstract
A palladium-catalyzed asymmetric (4 + 3) cycloaddition of N-2,2,2-trifluoroethylisatin ketimines with 2-methylidenetrimethylene carbonate was reported to enantioselectively afford trifluoromethylated spirooxindoles in moderate yields and good to excellent ee values. Experimentally, the reaction proceeded smoothly in the absence of a Brønsted base in a one-pot manner, which paved a way for efficient and cost-effective construction of optically pure trifluoromethylated medium-sized rings.
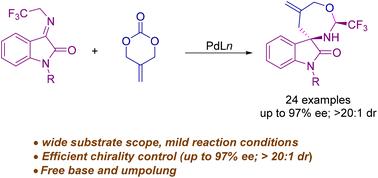
钯催化的N-2,2,2-三氟乙基isatin酮亚胺的不对称(4+3)环加成:获得光学活性螺环吲哚†
报道了钯催化的N-2,2,2-三氟乙基isatin酮亚胺与2-亚甲基三亚甲基碳酸酯的不对称(4+3)环加成反应,以中等产率和良好至优异的ee值对映选择性地得到三氟甲基螺环吲哚。在实验上,反应在没有Brønsted碱的情况下以一锅法顺利进行,这为高效和经济高效地构建光学纯三氟甲基化中型环铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


