Cristina A. Nadalutti, Samuel H. Wilson
{"title":"Using Human Primary Foreskin Fibroblasts to Study Cellular Damage and Mitochondrial Dysfunction","authors":"Cristina A. Nadalutti, Samuel H. Wilson","doi":"10.1002/cptx.99","DOIUrl":null,"url":null,"abstract":"<p>Several cell lines of different origin are routinely used in research and drug development as important models to study human health and disease. Studying cells in culture represents an easy and convenient tool to approach complex biological questions, but the disadvantage is that they may not necessarily reflect what is effectively occurring in vivo. Human primary cells can help address this limitation, as they are isolated directly from human biological samples and can preserve the morphological and functional features of their tissue of origin. In addition, these can offer more relevant data and better solutions to investigators because they are not genetically manipulated. Human foreskin tissue discarded after surgery, for instance, represents a precious source for isolating such cells, including human foreskin fibroblasts (FSK), which are used in several areas of research and medicine. The overall health of cells is determined by the mitochondria. Alterations of cellular metabolism and cell death pathways depend, in part, on the number, size, distribution, and structure of mitochondria, and these can change under different cellular and pathological conditions. This highlights the need to develop accurate approaches to study mitochondria and evaluate their function. Here, we describe three easy, step-by-step protocols to study cellular viability and mitochondrial functionality in FSK. We describe how to use circumcision tissue obtained from the clinic to isolate FSK cells by mechanical and enzymatic disaggregation, how to use a cationic dye, crystal violet, which is retained by proliferating cells, to determine cell viability, and how to prepare samples to assess the metabolic status of cells by evaluating different mitochondrial parameters with transmission electron microscopy. We have successfully used the approaches outlined here to recapitulate physiological conditions in these cells in order to study the effects of increased intracellular levels of formaldehyde. © 2020 U.S. Government.</p><p><b>Basic Protocol 1</b>: Isolation and maintenance of human primary foreskin fibroblasts (FSK)</p><p><b>Basic Protocol 2</b>: Determination of cell viability by crystal violet staining</p><p><b>Basic Protocol 3</b>: Transmission electron microscopy to study cellular damage and mitochondrial dysfunction</p>","PeriodicalId":72743,"journal":{"name":"Current protocols in toxicology","volume":"86 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-11-17","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cptx.99","citationCount":"3","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols in toxicology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cptx.99","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 3
Abstract
Several cell lines of different origin are routinely used in research and drug development as important models to study human health and disease. Studying cells in culture represents an easy and convenient tool to approach complex biological questions, but the disadvantage is that they may not necessarily reflect what is effectively occurring in vivo. Human primary cells can help address this limitation, as they are isolated directly from human biological samples and can preserve the morphological and functional features of their tissue of origin. In addition, these can offer more relevant data and better solutions to investigators because they are not genetically manipulated. Human foreskin tissue discarded after surgery, for instance, represents a precious source for isolating such cells, including human foreskin fibroblasts (FSK), which are used in several areas of research and medicine. The overall health of cells is determined by the mitochondria. Alterations of cellular metabolism and cell death pathways depend, in part, on the number, size, distribution, and structure of mitochondria, and these can change under different cellular and pathological conditions. This highlights the need to develop accurate approaches to study mitochondria and evaluate their function. Here, we describe three easy, step-by-step protocols to study cellular viability and mitochondrial functionality in FSK. We describe how to use circumcision tissue obtained from the clinic to isolate FSK cells by mechanical and enzymatic disaggregation, how to use a cationic dye, crystal violet, which is retained by proliferating cells, to determine cell viability, and how to prepare samples to assess the metabolic status of cells by evaluating different mitochondrial parameters with transmission electron microscopy. We have successfully used the approaches outlined here to recapitulate physiological conditions in these cells in order to study the effects of increased intracellular levels of formaldehyde. © 2020 U.S. Government.
Basic Protocol 1: Isolation and maintenance of human primary foreskin fibroblasts (FSK)
Basic Protocol 2: Determination of cell viability by crystal violet staining
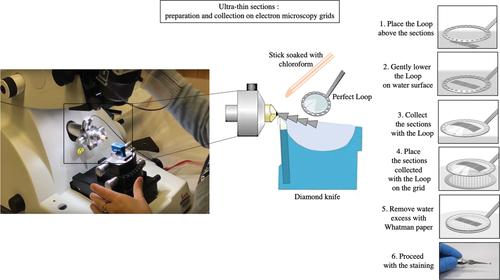
Basic Protocol 3: Transmission electron microscopy to study cellular damage and mitochondrial dysfunction
利用人原代包皮成纤维细胞研究细胞损伤和线粒体功能障碍
不同来源的几种细胞系通常用于研究和药物开发,作为研究人类健康和疾病的重要模型。研究培养细胞是解决复杂生物学问题的一种简单方便的工具,但缺点是它们不一定反映体内有效发生的情况。人类原代细胞可以帮助解决这一限制,因为它们直接从人类生物样本中分离出来,并且可以保留其原始组织的形态和功能特征。此外,这些可以为调查人员提供更相关的数据和更好的解决方案,因为它们不是基因操纵的。例如,手术后丢弃的人类包皮组织是分离此类细胞的宝贵来源,包括人类包皮成纤维细胞(FSK),它被用于多个研究和医学领域。细胞的整体健康是由线粒体决定的。细胞代谢和细胞死亡途径的改变部分取决于线粒体的数量、大小、分布和结构,这些可以在不同的细胞和病理条件下发生变化。这突出了开发准确的方法来研究线粒体并评估其功能的必要性。在这里,我们描述了三个简单的,一步一步的协议来研究细胞活力和线粒体功能在FSK。我们描述了如何使用从临床获得的包皮环切组织,通过机械和酶解分离FSK细胞,如何使用阳离子染料,结晶紫,这是由增殖细胞保留,以确定细胞活力,以及如何准备样品,以评估细胞的代谢状态,通过评估不同的线粒体参数与透射电子显微镜。我们已经成功地使用了这里概述的方法来概括这些细胞中的生理条件,以便研究细胞内甲醛水平增加的影响。©2020美国政府。基本方案1:人原代包皮成纤维细胞(FSK)的分离和维持基本方案2:结晶紫染色测定细胞活力基本方案3:透射电子显微镜研究细胞损伤和线粒体功能障碍
本文章由计算机程序翻译,如有差异,请以英文原文为准。


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


