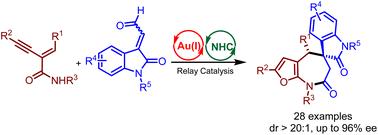
Asymmetric synthesis of spirofuro[2,3-b]azepine-5,3′-indoline derivatives via cycloisomerization/[4 + 3] annulation process under Au/N-heterocyclic carbene relay catalysis†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00539a
引用次数: 0
Abstract
A variety of enantioenriched spirofuro[2,3-b]azepine-5,3′-indoline derivatives (dr > 20 : 1, up to 96% ee) were facilely synthesized through cycloisomerization/asymmetric formal [4 + 3] cycloaddition reactions of enyne-amides with isatin-derived enals under gold(i)/chiral N-heterocyclic carbene (NHC) relay catalysis. The method is practical and useful because of the convenient one-step procedure, high stereoselectivity, and good functional-group tolerance.
Au/N-杂环卡宾中继催化下通过环异构化/[4+3]环化过程不对称合成螺呋并[2,3-b]氮杂平-5,3′-吲哚啉衍生物†
多种对映体富集的螺呋[2,3-b]氮杂平-5,3′-吲哚啉衍生物(dr>;20 : 1,高达96%ee)在金(i)/手性N-杂环卡宾(NHC)中继催化下,通过烯炔酰胺与靛红衍生的烯醛的环异构化/不对称形式[4+3]环加成反应,容易地合成。该方法具有一步操作简便、立体选择性强、官能团耐受性好等优点,具有实用性和实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


