Ru(ii)-catalyzed C–H alkynylation of ferrocenes with bromoalkynes directed by carboxamide groups†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00069a
引用次数: 0
Abstract
An efficient synthesis of ferrocene derivatives via Ru(ii)-catalyzed direct selective C–H mono-alkynylation with easily accessible bromoalkyne compounds under the influence of weakly coordinating amide groups has been presented. This protocol provides an approach for accessing various ferrocene derivatives with easily transformed alkynes using carboxamides, giving alkynylated ferrocenes in up to 89% yield.
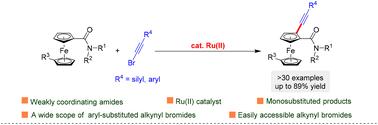
Ru(ii)催化的二茂铁与溴炔烃的C–H炔化反应,由羧酰胺基团指导†
在弱配位酰胺基团的影响下,通过Ru(ii)催化的与容易获得的溴炔烃化合物的直接选择性C–H单炔化,有效地合成了二茂铁衍生物。该方案提供了一种使用羧酰胺用容易转化的炔烃制备各种二茂铁衍生物的方法,得到高达89%产率的炔基化二茂铁。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


