Visible light mediated regioselective 1,3-oxylallylation of aryl cyclopropanes under redox-neutral conditions†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00281k
引用次数: 0
Abstract
A photoredox catalysed 1,3-oxylallylation of aryl cyclopropanes was accomplished by reaction with carboxylic acids and allyl sulfones. The redox-neutral reaction proceeded in a highly regioselective manner under mild conditions with good functional group compatibility. Simple operation and successful application to the late-stage functionalization of several natural product and pharmaceutical molecule related carboxylic acids add extra merits to the current protocol.
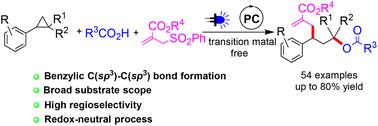
可见光介导的芳基环丙烷在氧化还原中性条件下的区域选择性1,3-氧烯丙基化†
通过与羧酸和烯丙基砜的反应,实现了芳基环丙烷的光氧化还原催化1,3-氧烯丙基化。氧化还原中性反应在具有良好官能团相容性的温和条件下以高度区域选择性的方式进行。简单的操作和成功应用于几种天然产物和药物分子相关羧酸的后期功能化为当前方案增加了额外的优点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


