Ben F. Brian 4th, Candace R. Guerrero, Tanya S. Freedman
{"title":"Immunopharmacology and Quantitative Analysis of Tyrosine Kinase Signaling","authors":"Ben F. Brian 4th, Candace R. Guerrero, Tanya S. Freedman","doi":"10.1002/cpim.104","DOIUrl":null,"url":null,"abstract":"<p>In this article we describe the use of pharmacological and genetic tools coupled with immunoblotting (Western blotting) and targeted mass spectrometry to quantify immune signaling and cell activation mediated by tyrosine kinases. Transfer of the ATP γ phosphate to a protein tyrosine residue activates signaling cascades regulating the differentiation, survival, and effector functions of all cells, with unique roles in immune antigen receptor, polarization, and other signaling pathways. Defining the substrates and scaffolding interactions of tyrosine kinases is critical for revealing and therapeutically manipulating mechanisms of immune regulation. Quantitative analysis of the amplitude and kinetics of these effects is becoming ever more accessible experimentally and increasingly important for predicting complex downstream effects of therapeutics and for building computational models. Secondarily, quantitative analysis is increasingly expected by reviewers and journal editors, and statistical analysis of biological replicates can bolster claims of experimental rigor and reproducibility. Here we outline methods for perturbing tyrosine kinase activity in cells and quantifying protein phosphorylation in lysates and immunoprecipitates. The immunoblotting techniques are a guide to probing the dynamics of protein abundance, protein–protein interactions, and changes in post-translational modification. Immunoprecipitated protein complexes can also be subjected to targeted mass spectrometry to probe novel sites of modification and multiply modified or understudied proteins that cannot be resolved by immunoblotting. Together, these protocols form a framework for identifying the unique contributions of tyrosine kinases to cell activation and elucidating the mechanisms governing immune cell regulation in health and disease. © 2020 The Authors.</p><p><b>Basic Protocol 1</b>: Quantifying protein phosphorylation via immunoblotting and near-infrared imaging</p><p><b>Alternate Protocol</b>: Visualizing immunoblots using chemiluminescence</p><p><b>Basic Protocol 2</b>: Enriching target proteins and isolation of protein complexes by immunoprecipitation</p><p><b>Support Protocol</b>: Covalent conjugation of antibodies to functionalized beads</p><p><b>Basic Protocol 3</b>: Quantifying proteins and post-translational modifications by targeted mass spectrometry</p>","PeriodicalId":10733,"journal":{"name":"Current Protocols in Immunology","volume":"130 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-09-15","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpim.104","citationCount":"6","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols in Immunology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpim.104","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"Immunology and Microbiology","Score":null,"Total":0}
引用次数: 6
Abstract
In this article we describe the use of pharmacological and genetic tools coupled with immunoblotting (Western blotting) and targeted mass spectrometry to quantify immune signaling and cell activation mediated by tyrosine kinases. Transfer of the ATP γ phosphate to a protein tyrosine residue activates signaling cascades regulating the differentiation, survival, and effector functions of all cells, with unique roles in immune antigen receptor, polarization, and other signaling pathways. Defining the substrates and scaffolding interactions of tyrosine kinases is critical for revealing and therapeutically manipulating mechanisms of immune regulation. Quantitative analysis of the amplitude and kinetics of these effects is becoming ever more accessible experimentally and increasingly important for predicting complex downstream effects of therapeutics and for building computational models. Secondarily, quantitative analysis is increasingly expected by reviewers and journal editors, and statistical analysis of biological replicates can bolster claims of experimental rigor and reproducibility. Here we outline methods for perturbing tyrosine kinase activity in cells and quantifying protein phosphorylation in lysates and immunoprecipitates. The immunoblotting techniques are a guide to probing the dynamics of protein abundance, protein–protein interactions, and changes in post-translational modification. Immunoprecipitated protein complexes can also be subjected to targeted mass spectrometry to probe novel sites of modification and multiply modified or understudied proteins that cannot be resolved by immunoblotting. Together, these protocols form a framework for identifying the unique contributions of tyrosine kinases to cell activation and elucidating the mechanisms governing immune cell regulation in health and disease. © 2020 The Authors.
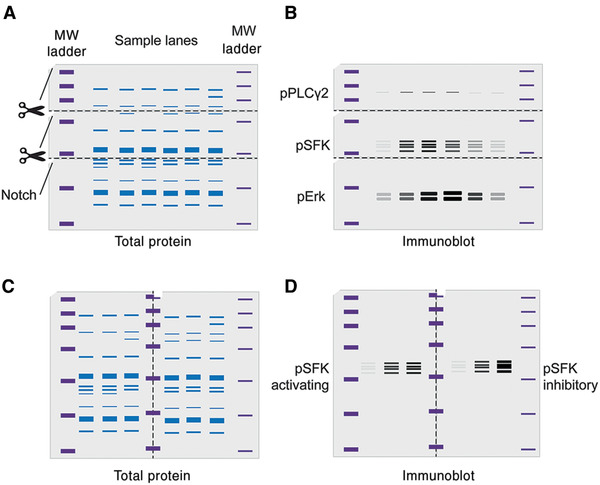
Basic Protocol 1: Quantifying protein phosphorylation via immunoblotting and near-infrared imaging
Alternate Protocol: Visualizing immunoblots using chemiluminescence
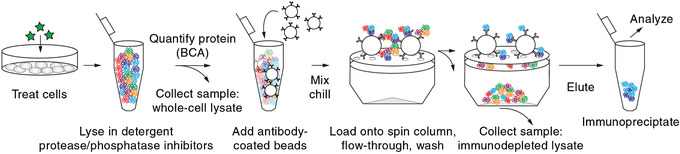
Basic Protocol 2: Enriching target proteins and isolation of protein complexes by immunoprecipitation
Support Protocol: Covalent conjugation of antibodies to functionalized beads
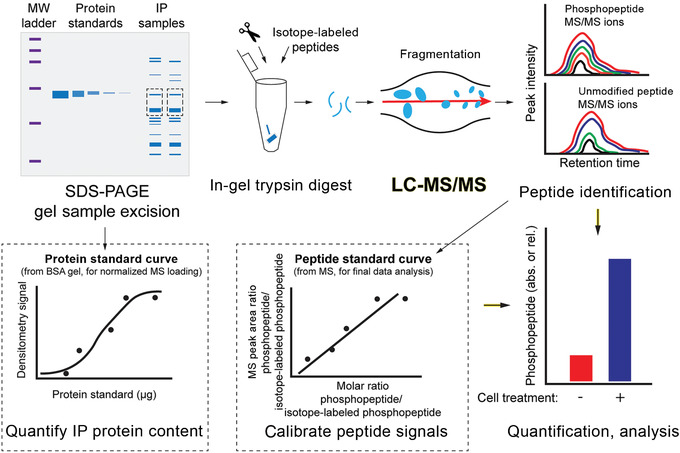
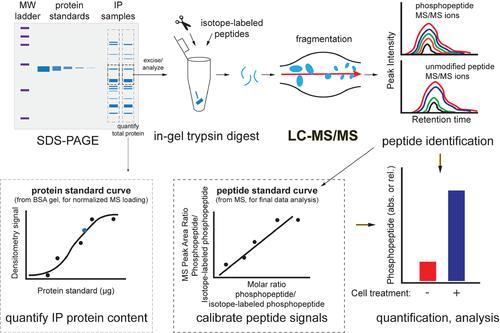
Basic Protocol 3: Quantifying proteins and post-translational modifications by targeted mass spectrometry
酪氨酸激酶信号的免疫药理学和定量分析
在这篇文章中,我们描述了使用药理学和遗传学工具结合免疫印迹(Western blotting)和靶向质谱法来定量免疫信号和酪氨酸激酶介导的细胞活化。ATP γ磷酸转移到蛋白酪氨酸残基激活信号级联,调节所有细胞的分化、存活和效应功能,在免疫抗原受体、极化和其他信号通路中具有独特的作用。确定酪氨酸激酶的底物和支架相互作用对于揭示和治疗操纵免疫调节机制至关重要。这些效应的幅度和动力学的定量分析在实验上变得越来越容易获得,并且对于预测治疗的复杂下游效应和建立计算模型越来越重要。其次,审稿人和期刊编辑越来越期望定量分析,生物重复的统计分析可以支持实验严谨性和可重复性的主张。在这里,我们概述了在细胞中干扰酪氨酸激酶活性和定量蛋白磷酸化裂解物和免疫沉淀的方法。免疫印迹技术是探测蛋白质丰度、蛋白质-蛋白质相互作用和翻译后修饰变化动态的指南。免疫沉淀蛋白复合物也可以采用靶向质谱法来探测新的修饰位点,并将不能通过免疫印迹法解决的修饰或未充分研究的蛋白相乘。总之,这些方案形成了一个框架,用于识别酪氨酸激酶对细胞激活的独特贡献,并阐明健康和疾病中免疫细胞调节的机制。©2020作者。基本方案1:通过免疫印迹和近红外成像定量蛋白磷酸化替代方案:使用化学发光可视化免疫印迹基本方案2:通过免疫沉淀富集靶蛋白和分离蛋白复合物支持方案:抗体与功能化珠的共价偶联基本方案3:通过靶向质谱法定量蛋白和翻译后修饰
本文章由计算机程序翻译,如有差异,请以英文原文为准。





 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


