Ni-catalyzed regioselective hydrobenzylation of alkenes to afford C(sp3)–C(sp3) bonds using BH3 as a reductant†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d2qo01510b
引用次数: 1
Abstract
The nickel-catalyzed reductive olefin hydrocarbonation reaction is one of the powerful tools for the formation of C(sp3)–C bonds. However, the reductive coupling of alkenes with benzyl halides to afford alkyl–benzyl products has rarely been studied using the well-established Ni/SiH methods. Herein, we report a method of Ni-catalyzed regioselective hydrobenzylation of unactivated alkenes to afford anti-Markovnikov products using BH3 as a reductant. This method exhibits good functional group tolerance, a wide scope and high regioselectivity. The mild conditions enable the coupling of terminal and internal alkenes with primary and secondary benzyl halides.
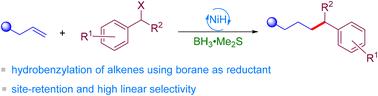
使用BH3作为还原剂,Ni催化烯烃的区域选择性加氢苄基化以提供C(sp3)–C(sp三)键†
镍催化的还原性烯烃加氢反应是形成C(sp3)–C键的有力工具之一。然而,很少使用公认的Ni/SiH方法研究烯烃与苄基卤化物的还原偶联以提供烷基-苄基产物。在此,我们报道了一种使用BH3作为还原剂对未活化的烯烃进行Ni催化的区域选择性加氢苄基化以制备抗Markovnikov产物的方法。该方法显示出良好的官能团耐受性、宽的范围和高的区域选择性。温和的条件使得末端和内部烯烃能够与伯和仲苄基卤化物偶联。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


