Fe/S cluster catalyzed cascade cyclization of N,S-1,6-enynes for the synthesis of thieno[3,4-b]indoles†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00697b
引用次数: 0
Abstract
Fe/S cluster catalyzed radical cascade cyclization of alkylthio-functionalized o-anilide-embedded N,S-1,6-enynes to afford thieno[3,4-b]indoles is developed. The cycloisomerization strategy offers a straightforward route to 4H-thieno[3,4-b]indoles through the 1,2-sulfur transfer and Csp3–S bond cleavage, with the formation of both an indole ring and a fused thiophene ring in one pot. In this cascade, the element sulfur acts as the oxidant to induce the single electron transfer (SET) process.
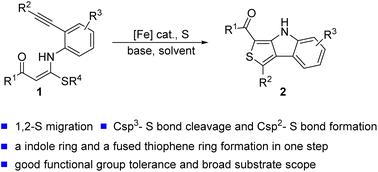
Fe/S簇合物催化N,S-1,6-炔烃级联环化合成噻吩并[3,4-b]吲哚†
Fe/S簇合物催化烷硫基官能化邻苯胺嵌入N,S-1,6-炔烃的自由基级联环化反应,得到噻吩并[3,4-b]吲哚。环异构化策略通过1,2-硫转移和Csp3–S键断裂提供了一条直接制备4H-噻吩并[3,4-b]吲哚的途径,在一锅中形成吲哚环和稠合噻吩环。在这个级联中,元素硫作为氧化剂来诱导单电子转移(SET)过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


