Photocatalytic decarboxylative selenocyanation of 2-aryloxy and 2-aryl carboxylic acids with N-selenocyanatophthalimide†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00347g
引用次数: 0
Abstract
A visible-light-induced decarboxylative selenocyanation reaction of aliphatic carboxylic acids for the formation of C(sp3)–SeCN bonds has been developed. The electrophilic N-selenocyanatophthalimide and a pyrylium salt were applied as a SeCN radical precursor and an organic photocatalyst, respectively. The transformation could proceed smoothly without transition metals, oxidants and additional bases, affording the corresponding alkyl selenocyanates in moderate to good yields. This method is characterized by its simplicity, wide substrate scope and mild conditions.
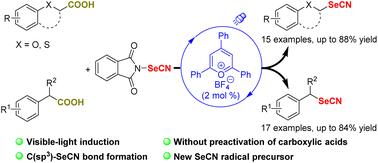
2-芳氧基和2-芳基羧酸与N-硒代氰邻苯二甲酰亚胺的光催化脱羧硒代氰†
开发了一种可见光诱导的脂肪族羧酸脱羧硒代氰反应,用于形成C(sp3)–SeCN键。亲电N-硒代氰基邻苯二甲酰亚胺和丙酮酸盐分别用作SeCN自由基前体和有机光催化剂。在没有过渡金属、氧化剂和额外碱的情况下,转化可以顺利进行,以中等至良好的产率提供相应的烷基硒代氰酸酯。该方法具有简单、底物范围广、条件温和等特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


