The Effect of Lithium on Inflammation-Associated Genes in Lipopolysaccharide-Activated Raw 264.7 Macrophages.
IF 2
Q3 IMMUNOLOGY
International Journal of Inflammation
Pub Date : 2020-07-25
eCollection Date: 2020-01-01
DOI:10.1155/2020/8340195
引用次数: 4
Abstract
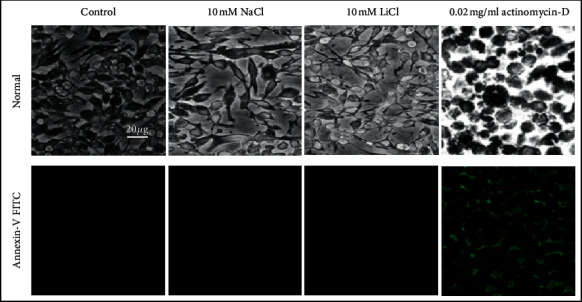
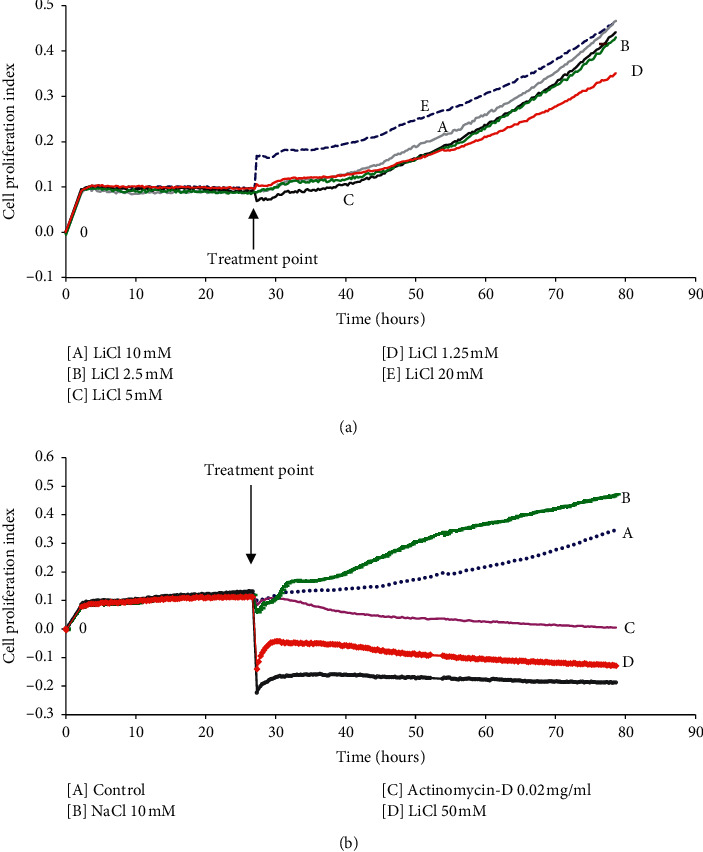
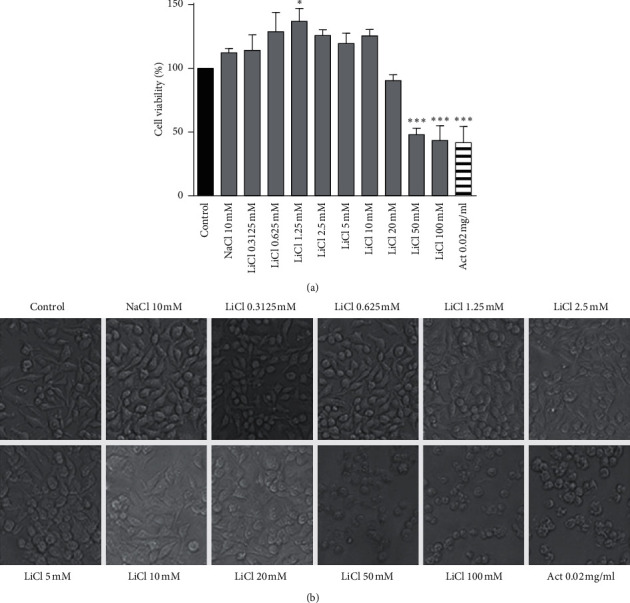
Lithium remains the preferred Food and Drug Administration- (FDA-) approved psychiatric drug for treatment of bipolar disorders since its medical establishment more than half a century ago. Recent studies revealed a promising role for lithium in the regulation of inflammation, oxidative stress, and neurodegeneration albeit unclear about its exact mode of action. Thus, the intention of this study is to delineate the regulatory mechanisms of lithium on oxidative stress in lipopolysaccharide- (LPS-) activated macrophages by evaluating its effects on nuclear factor-κB (NF-κB) activity and mRNA expression of multiple oxidative stress-related NF-κB genes. Raw 264.7 macrophages were treated with up to 10 mM lithium, and no change in cell proliferation, viability, growth, and cell adhesion was observed in real time. Pretreatment with low doses of lithium was shown to reduce nitric oxide (NO) production in LPS-activated macrophages. A reduced internal H2DCFDA fluorescence intensity, indicative of reduced reactive oxygen species (ROS) production, was observed in LPS-activated Raw 264.7 macrophages treated with lithium. Lithium has been shown to lower the production of the chemokine RANTES; furthermore, this inhibitory action of lithium has been suggested to be independent of glycogen synthase kinase-3 β (GSK3β) activity. It is shown here that lithium modulates the expression of several inflammatory genes including IκB-α, TRAF3, Tollip, and NF-κB1/p50 which are regulators of the NF-κB pathway. Moreover, lithium inhibits NF-κB activity by lowering nuclear translocation of NF-κB in LPS-activated macrophages. This is the first study to associate Tollip, Traf-3, and IκB-α mRNA expression with lithium effect on NF-κB activity in LPS-activated Raw 264.7 macrophages. Although these effects were obtained using extratherapeutic concentrations of lithium, results of this study provide useful information towards understanding the mode of action of lithium. This study associates lithium with reduced oxidative stress in LPS-activated Raw 264.7 macrophages and further suggests candidate molecular targets for the regulation of oxidative stress-related diseases using lithium beyond bipolar disorders.
锂对脂多糖活化生264.7巨噬细胞炎症相关基因的影响。
自半个多世纪前锂药物问世以来,它一直是美国食品和药物管理局(FDA)批准的治疗双相情感障碍的首选精神药物。最近的研究揭示了锂在炎症、氧化应激和神经退行性变的调节中有希望的作用,尽管尚不清楚其确切的作用方式。因此,本研究旨在通过评价锂对核因子-κ b (NF-κ b)活性和多种氧化应激相关NF-κ b基因mRNA表达的影响,揭示锂对脂多糖(LPS)活化巨噬细胞氧化应激的调控机制。未处理的264.7巨噬细胞以高达10 mM的锂处理,实时观察细胞增殖、活力、生长和细胞粘附无变化。低剂量锂预处理可减少lps激活的巨噬细胞中一氧化氮(NO)的产生。在锂处理的lps活化的Raw 264.7巨噬细胞中,观察到内部H2DCFDA荧光强度降低,表明活性氧(ROS)的产生减少。锂已被证明可以降低趋化因子RANTES的产生;此外,锂的这种抑制作用被认为与糖原合成酶激酶-3 β (GSK3β)活性无关。研究表明,锂可以调节几种炎症基因的表达,包括i -κB -α、TRAF3、Tollip和NF-κB1/p50,这些基因是NF-κB通路的调节因子。此外,锂通过降低lps活化的巨噬细胞中NF-κB的核易位来抑制NF-κB活性。这是第一个将Tollip、trf -3和i -κB -α mRNA表达与锂对lps活化的Raw 264.7巨噬细胞NF-κB活性的影响联系起来的研究。虽然这些效应是通过治疗外浓度的锂获得的,但本研究的结果为理解锂的作用模式提供了有用的信息。这项研究将锂与lps激活的Raw 264.7巨噬细胞氧化应激的降低联系起来,并进一步提出了使用锂来调节双相情感障碍以外的氧化应激相关疾病的候选分子靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Inflammation
IMMUNOLOGY-
CiteScore
3.80
自引率
0.00%
发文量
16
审稿时长
16 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


