Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 55
Abstract
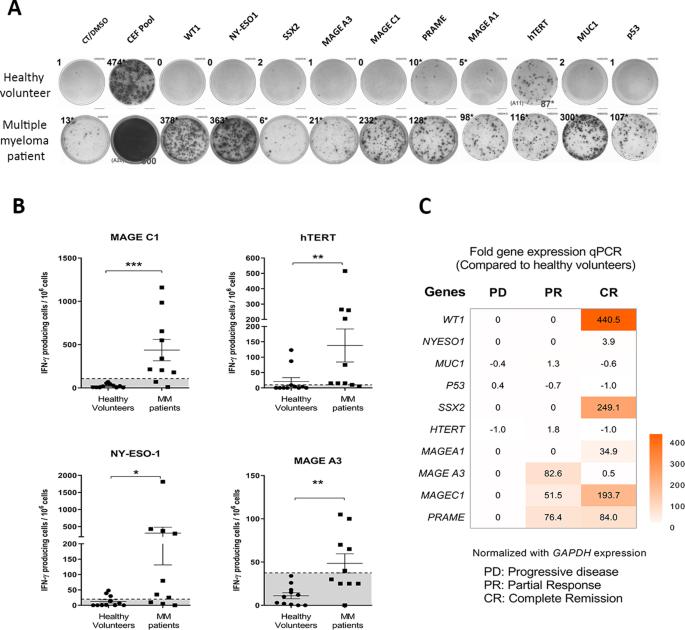
Oncolytic virus therapy leads to immunogenic death of virus-infected tumor cells and this has been shown in preclinical models to enhance the cytotoxic T-lymphocyte response against tumor-associated antigens (TAAs), leading to killing of uninfected tumor cells. To investigate whether oncolytic virotherapy can increase immune responses to tumor antigens in human subjects, we studied T-cell responses against a panel of known myeloma TAAs using PBMC samples obtained from ten myeloma patients before and after systemic administration of an oncolytic measles virus encoding sodium iodide symporter (MV-NIS). Despite their prior exposures to multiple immunosuppressive antimyeloma treatment regimens, T-cell responses to some of the TAAs were detectable even before measles virotherapy. Measurable baseline T-cell responses against MAGE-C1 and hTERT were present. Furthermore, MV-NIS treatment significantly (P < 0.05) increased T-cell responses against MAGE-C1 and MAGE-A3. Interestingly, one patient who achieved complete remission after MV-NIS therapy had strong baseline T-cell responses both to measles virus proteins and to eight of the ten tested TAAs. Our data demonstrate that oncolytic virotherapy can function as an antigen agnostic vaccine, increasing cytotoxic T-lymphocyte responses against TAAs in patients with multiple myeloma, providing a basis for continued exploration of this modality in combination with immune checkpoint blockade.
肿瘤溶解性麻疹病毒疗法可增强多发性骨髓瘤患者的肿瘤抗原特异性 T 细胞反应
溶瘤病毒疗法会导致受病毒感染的肿瘤细胞免疫性死亡,临床前模型显示,这种疗法会增强细胞毒性T淋巴细胞对肿瘤相关抗原(TAAs)的反应,从而杀死未感染的肿瘤细胞。为了研究溶瘤病毒疗法是否能增强人体对肿瘤抗原的免疫反应,我们利用十名骨髓瘤患者在全身注射编码碘化钠合酶的溶瘤麻疹病毒(MV-NIS)前后获得的白细胞介素样本,研究了T细胞对一组已知骨髓瘤TAAs的反应。尽管他们之前接受过多种免疫抑制抗骨髓瘤治疗方案,但即使在麻疹病毒治疗之前,也能检测到对某些 TAAs 的 T 细胞反应。针对 MAGE-C1 和 hTERT 的可测量基线 T 细胞反应已经出现。此外,MV-NIS 治疗显著增加了针对 MAGE-C1 和 MAGE-A3 的 T 细胞反应(P < 0.05)。有趣的是,一名经 MV-NIS 治疗后获得完全缓解的患者对麻疹病毒蛋白和 10 种测试 TAAs 中的 8 种都有很强的基线 T 细胞反应。我们的数据表明,溶瘤病毒疗法可以作为抗原无关疫苗发挥作用,提高多发性骨髓瘤患者针对TAAs的细胞毒性T淋巴细胞反应,为继续探索这种疗法与免疫检查点阻断疗法的结合提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


