Chlorine disinfection promotes the exchange of antibiotic resistance genes across bacterial genera by natural transformation
IF 10.8
1区 环境科学与生态学
Q1 ECOLOGY
引用次数: 155
Abstract
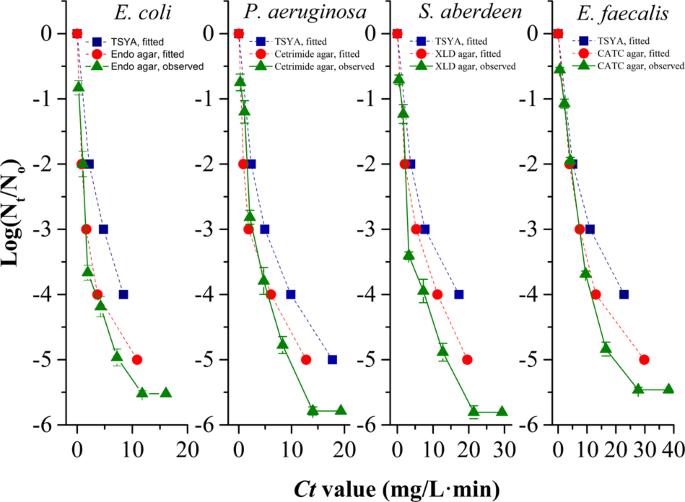
Chlorine disinfection to drinking water plays an important role in preventing and controlling waterborne disease outbreaks globally. Nevertheless, little is known about why it enriches the antibiotic resistance genes (ARGs) in bacteria after chlorination. Here, ARGs released from killed antibiotic-resistant bacteria (ARB), and culturable chlorine-injured bacteria produced in the chlorination process as the recipient, were investigated to determine their contribution to the horizontal transfer of ARGs during disinfection treatment. We discovered Escherichia coli, Salmonella aberdeen, Pseudomonas aeruginosa and Enterococcus faecalis showed diverse resistance to sodium hypochlorite, and transferable RP4 could be released from killed sensitive donor consistently. Meanwhile, the survival of chlorine-tolerant injured bacteria with enhanced cell membrane permeabilisation and a strong oxidative stress-response demonstrated that a physiologically competent cell could be transferred by RP4 with an improved transformation frequency of up to 550 times compared with the corresponding untreated bacteria. Furthermore, the water quality factors involving chemical oxygen demand (CODMn), ammonium nitrogen and metal ions (Ca2+ and K+) could significantly promote above transformation frequency of released RP4 into injured E. faecalis. Our findings demonstrated that the chlorination process promoted the horizontal transfer of plasmids by natural transformation, which resulted in the exchange of ARGs across bacterial genera and the emergence of new ARB, as well as the transfer of chlorine-injured opportunistic pathogen from non-ARB to ARB. Considering that the transfer elements were quite resistant to degradation through disinfection, this situation poses a potential risk to public health.
氯消毒通过自然转化促进细菌属间抗生素抗性基因的交换。
饮用水氯消毒在预防和控制全球水传播疾病暴发中发挥着重要作用。然而,人们对氯化后细菌中抗生素抗性基因(ARGs)富集的原因知之甚少。本研究研究了杀死的耐药细菌(ARB)释放的ARGs,以及氯化过程中产生的可培养氯损伤细菌作为受体,以确定它们在消毒处理过程中对ARGs水平转移的贡献。我们发现大肠杆菌、阿伯丁沙门氏菌、铜绿假单胞菌和粪肠球菌对次氯酸钠具有不同的抗性,并且在被杀死的敏感供体中一致释放可转移的RP4。与此同时,耐氯损伤细菌的细胞膜通透性增强,氧化应激反应强,其存活表明,与未处理的细菌相比,RP4可以将生理上胜任的细胞转移,其转化频率提高了550倍。化学需氧量(CODMn)、铵态氮(铵态氮)和金属离子(Ca2+和K+)等水质因子能显著促进上述释放的RP4向受伤粪肠杆菌转化的频率。我们的研究结果表明,氯化过程通过自然转化促进了质粒的水平转移,从而导致arg在细菌属之间的交换和新的ARB的出现,以及氯损伤的机会病原体从非ARB向ARB转移。考虑到转移元素通过消毒对降解具有很强的抵抗力,这种情况对公共卫生构成潜在风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ISME Journal
环境科学-生态学
CiteScore
22.10
自引率
2.70%
发文量
171
审稿时长
2.6 months
期刊介绍:
The ISME Journal covers the diverse and integrated areas of microbial ecology. We encourage contributions that represent major advances for the study of microbial ecosystems, communities, and interactions of microorganisms in the environment. Articles in The ISME Journal describe pioneering discoveries of wide appeal that enhance our understanding of functional and mechanistic relationships among microorganisms, their communities, and their habitats.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


