Incidence of CXCR4 tropism and CCR5-tropic resistance in treatment-experienced participants receiving maraviroc in the 48-week MOTIVATE 1 and 2 trials.
Q2 Pharmacology, Toxicology and Pharmaceutics
引用次数: 0
Abstract
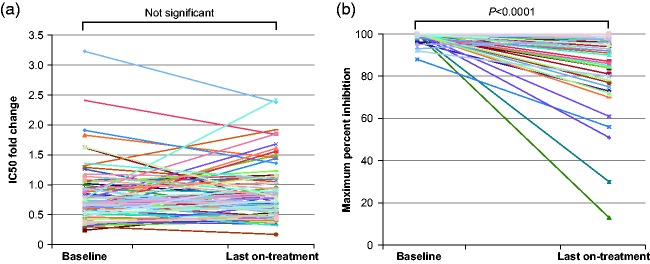
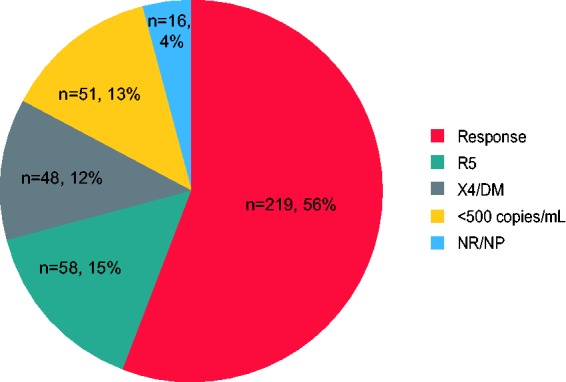
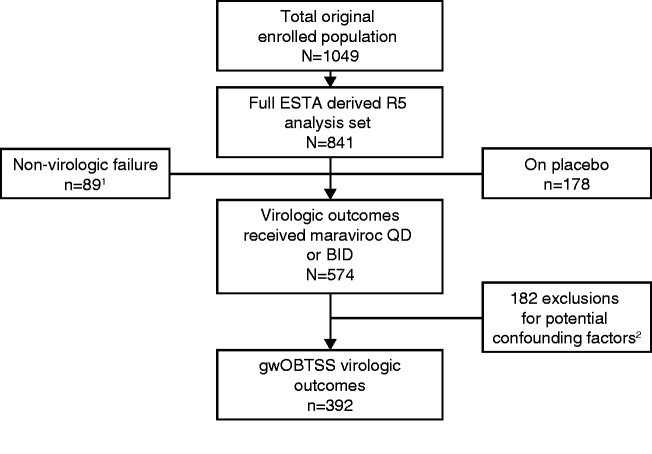
Maraviroc blocks HIV-1 entry into CD4+ cells by interrupting the interaction between viral gp120 and cell-surface CCR5. Resistance to CCR5 antagonist–mediated inhibition can develop by unmasking pre-existing CXCR4-using virus or through selection of CCR5-tropic resistant virus, characterized by plateaus in maximum percent inhibition <95%. Here, we examine viral escape in maraviroc-treated participants during virologic failure through Week 48 in the MOTIVATE 1 and 2 trials. Resistance was assessed relative to number of active drugs in participants’ optimized background therapy, pharmacokinetic adherence markers, Baseline demographic data, HIV-1 RNA and CD4+ counts. For participants with R5 virus confirmed (post hoc) at Screening, Baseline genotypic weighted optimized background therapy susceptibility scores (gwOBTSS) were assigned where possible. Through Week 48, 219/392 (56%) participants with an assigned gwOBTSS achieved a virologic response. Of those remaining, 48/392 (12%) had CXCR4-using virus; 58/392 (15%) had R5 virus (maraviroc sensitive: n = 35/392, 9%; maraviroc resistant: n = 18/392, 5%; undeterminable: n = 5/392, 1%) and 67/392 (17%) had no failure tropism result. When optimized background therapy provided limited support to maraviroc (gwOBTSS <2), 143/286 (50%) responded to therapy, while 76/106 (72%) participants with gwOBTSS ≥2 responded (p < 0.001). Resistance rates were highest for participants with gwOBTSS <2, accounting for 45/48 (94%) of total CXCR4-using emergence and 18/18 (100%) of total CCR5-tropic resistance. R5 viruses from participants with gwOBTSS ≥2 (n = 10) were exclusively maraviroc sensitive; five of these participants had pharmacokinetic and/or pill-count markers of non-adherence. When co-administered with a fully active background regimen, maraviroc did not readily generate resistance in the clinical setting. Trial registry name: ClinicalTrials.gov (https://clinicaltrials.gov/), NCT00098722 and NCT00098306
在48周的MOTIVATE 1和2试验中,接受过马拉韦洛克治疗的参与者中CXCR4向性和ccr5向性耐药的发生率
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Antiviral Chemistry and Chemotherapy
Pharmacology, Toxicology and Pharmaceutics-Pharmacology
CiteScore
5.20
自引率
0.00%
发文量
5
审稿时长
15 weeks
期刊介绍:
Antiviral Chemistry & Chemotherapy publishes the results of original research concerned with the biochemistry, mode of action, chemistry, pharmacology and virology of antiviral compounds. Manuscripts dealing with molecular biology, animal models and vaccines are welcome. The journal also publishes reviews, pointers, short communications and correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


