Cassava Metabolomics and Starch Quality
Q1 Agricultural and Biological Sciences
Laise Rosado-Souza, Laure C. David, Margit Drapal, Paul D. Fraser, Jörg Hofmann, Patrick A. W. Klemens, Frank Ludewig, H. Ekkehard Neuhaus, Toshihiro Obata, Laura Perez-Fons, Armin Schlereth, Uwe Sonnewald, Mark Stitt, Samuel C. Zeeman, Wolfgang Zierer, Alisdair R. Fernie
下载PDF
{"title":"Cassava Metabolomics and Starch Quality","authors":"Laise Rosado-Souza, Laure C. David, Margit Drapal, Paul D. Fraser, Jörg Hofmann, Patrick A. W. Klemens, Frank Ludewig, H. Ekkehard Neuhaus, Toshihiro Obata, Laura Perez-Fons, Armin Schlereth, Uwe Sonnewald, Mark Stitt, Samuel C. Zeeman, Wolfgang Zierer, Alisdair R. Fernie","doi":"10.1002/cppb.20102","DOIUrl":null,"url":null,"abstract":"<p>Cassava plays an important role as a staple food for more than 800 million people in the world due to its ability to maintain relatively high productivity even in nutrient-depleted soils. Even though cassava has been the focus of several breeding programs and has become a strong focus of research in the last few years, relatively little is currently known about its metabolism and metabolic composition in different tissues. In this article, the absolute content of sugars, organic acids, amino acids, phosphorylated intermediates, minerals, starch, carotenoids, chlorophylls, tocopherols, and total protein as well as starch quality is described based on multiple analytical techniques, with protocols specifically adjusted for material from different cassava tissues. Moreover, quantification of secondary metabolites relative to internal standards is presented using both non-targeted and targeted metabolomics approaches. The protocols have also been adjusted to apply to freeze-dried material in order to allow processing of field harvest samples that typically will require long-distance transport. © 2019 The Authors.</p><p><b>Basic Protocol 1</b>: Metabolic profiling by gas chromatography–mass spectrometry (GC-MS)</p><p><b>Support Protocol 1</b>: Preparation of freeze-dried cassava material</p><p><b>Support Protocol 2</b>: Preparation of standard compound mixtures for absolute quantification of metabolites by GC-MS</p><p><b>Support Protocol 3</b>: Preparation of retention-time standard mixture</p><p><b>Basic Protocol 2</b>: Determination of organic acids and phosphorylated intermediates by ion chromatography–mass spectrometry (IC-MS)</p><p><b>Support Protocol 4</b>: Preparation of standards and recovery experimental procedure</p><p><b>Basic Protocol 3</b>: Determination of soluble sugars, starch, and free amino acids</p><p><b>Alternate Protocol</b>: Determination of soluble sugars and starch</p><p><b>Basic Protocol 4</b>: Determination of anions</p><p><b>Basic Protocol 5</b>: Determination of elements</p><p><b>Basic Protocol 6</b>: Determination of total protein</p><p><b>Basic Protocol 7</b>: Determination of non-targeted and targeted secondary metabolites</p><p><b>Basic Protocol 8</b>: Determination of carotenoids, chlorophylls, and tocopherol</p><p><b>Basic Protocol 9</b>: Determination of starch quality</p>","PeriodicalId":10932,"journal":{"name":"Current protocols in plant biology","volume":"4 4","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2019-12-13","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cppb.20102","citationCount":"13","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols in plant biology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cppb.20102","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"Agricultural and Biological Sciences","Score":null,"Total":0}
引用次数: 13
引用
批量引用
Abstract
Cassava plays an important role as a staple food for more than 800 million people in the world due to its ability to maintain relatively high productivity even in nutrient-depleted soils. Even though cassava has been the focus of several breeding programs and has become a strong focus of research in the last few years, relatively little is currently known about its metabolism and metabolic composition in different tissues. In this article, the absolute content of sugars, organic acids, amino acids, phosphorylated intermediates, minerals, starch, carotenoids, chlorophylls, tocopherols, and total protein as well as starch quality is described based on multiple analytical techniques, with protocols specifically adjusted for material from different cassava tissues. Moreover, quantification of secondary metabolites relative to internal standards is presented using both non-targeted and targeted metabolomics approaches. The protocols have also been adjusted to apply to freeze-dried material in order to allow processing of field harvest samples that typically will require long-distance transport. © 2019 The Authors.
Basic Protocol 1 : Metabolic profiling by gas chromatography–mass spectrometry (GC-MS)
Support Protocol 1 : Preparation of freeze-dried cassava material
Support Protocol 2 : Preparation of standard compound mixtures for absolute quantification of metabolites by GC-MS
Support Protocol 3 : Preparation of retention-time standard mixture
Basic Protocol 2 : Determination of organic acids and phosphorylated intermediates by ion chromatography–mass spectrometry (IC-MS)
Support Protocol 4 : Preparation of standards and recovery experimental procedure
Basic Protocol 3 : Determination of soluble sugars, starch, and free amino acids
Alternate Protocol : Determination of soluble sugars and starch
Basic Protocol 4 : Determination of anions
Basic Protocol 5 : Determination of elements
Basic Protocol 6 : Determination of total protein
Basic Protocol 7 : Determination of non-targeted and targeted secondary metabolites
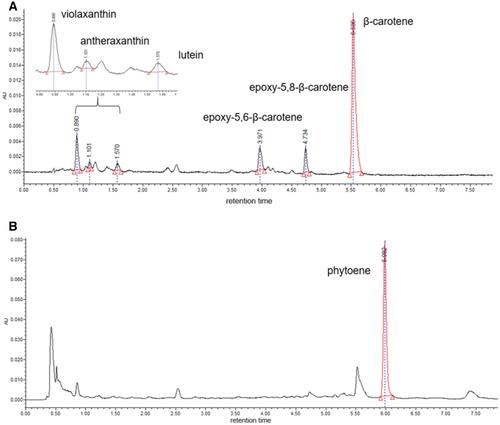
Basic Protocol 8 : Determination of carotenoids, chlorophylls, and tocopherol
Basic Protocol 9 : Determination of starch quality
木薯代谢组学与淀粉品质
木薯作为世界上8亿多人的主食发挥着重要作用,因为它即使在养分枯竭的土壤中也能保持相对较高的生产力。尽管木薯一直是几个育种计划的重点,并且在过去几年中已成为研究的重点,但目前对其在不同组织中的代谢和代谢组成所知相对较少。在本文中,糖、有机酸、氨基酸、磷酸化中间体、矿物质、淀粉、类胡萝卜素、叶绿素、生育酚和总蛋白的绝对含量以及淀粉质量基于多种分析技术进行了描述,并针对来自不同木薯组织的材料进行了专门调整。此外,二级代谢物相对于内部标准的量化提出了使用非靶向和靶向代谢组学方法。协议也进行了调整,适用于冻干材料,以便处理通常需要长途运输的田间收获样品。©2019作者。基本方案1:气相色谱-质谱(GC-MS)代谢谱分析支持方案1:制备冻干的cassava材料支持方案2:制备标准化合物混合物,用GC-MS对代谢物进行绝对定量支持方案3:制备保留时间标准混合物基本方案2:用离子色谱-质谱(IC-MS)测定有机酸和磷酸化中间体支持方案4:标准品的制备和回收实验程序基本方案3:可溶性糖、淀粉和游离氨基酸的测定替代方案:可溶性糖和淀粉的测定基本方案4:阴离子的测定基本方案5:元素的测定基本方案6:总蛋白的测定基本方案7:非靶向和靶向二级代谢产物的测定基本方案8:类胡萝卜素、叶绿素和生育酚的测定基本方案9:淀粉质量的测定
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


