Gauri Bhosale, Michael R. Duchen
{"title":"Investigating the Mitochondrial Permeability Transition Pore in Disease Phenotypes and Drug Screening","authors":"Gauri Bhosale, Michael R. Duchen","doi":"10.1002/cpph.59","DOIUrl":null,"url":null,"abstract":"<p>Mitochondria act as ‘sinks’ for Ca<sup>2+</sup> signaling, with mitochondrial Ca<sup>2+</sup> uptake linking physiological stimuli to increased ATP production. However, mitochondrial Ca<sup>2+</sup> overload can induce a cellular catastrophe by opening of the mitochondrial permeability transition pore (mPTP). This pore is a large conductance pathway in the inner mitochondrial membrane that causes bioenergetic collapse and appears to represent a final common path to cell death in many diseases. The role of the mPTP as a determinant of disease outcome is best established in ischemia/reperfusion injury in the heart, brain, and kidney, and it is also implicated in neurodegenerative disorders and muscular dystrophies. As the probability of pore opening can be modulated by drugs, it represents a useful pharmacological target for translational research in drug discovery. Described in this unit is a protocol utilizing isolated mitochondria to quantify this phenomenon and to develop a high-throughput platform for phenotypic screens for Ca<sup>2+</sup> dyshomeostasis. © 2019 The Authors. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.</p>","PeriodicalId":10871,"journal":{"name":"Current Protocols in Pharmacology","volume":"85 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2019-05-13","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpph.59","citationCount":"12","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols in Pharmacology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpph.59","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"Pharmacology, Toxicology and Pharmaceutics","Score":null,"Total":0}
引用次数: 12
Abstract
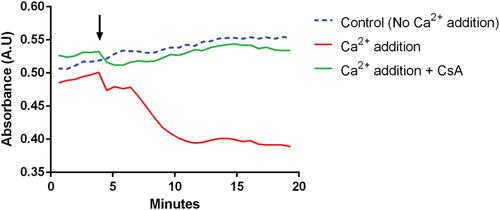
Mitochondria act as ‘sinks’ for Ca2+ signaling, with mitochondrial Ca2+ uptake linking physiological stimuli to increased ATP production. However, mitochondrial Ca2+ overload can induce a cellular catastrophe by opening of the mitochondrial permeability transition pore (mPTP). This pore is a large conductance pathway in the inner mitochondrial membrane that causes bioenergetic collapse and appears to represent a final common path to cell death in many diseases. The role of the mPTP as a determinant of disease outcome is best established in ischemia/reperfusion injury in the heart, brain, and kidney, and it is also implicated in neurodegenerative disorders and muscular dystrophies. As the probability of pore opening can be modulated by drugs, it represents a useful pharmacological target for translational research in drug discovery. Described in this unit is a protocol utilizing isolated mitochondria to quantify this phenomenon and to develop a high-throughput platform for phenotypic screens for Ca2+ dyshomeostasis. © 2019 The Authors. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
线粒体通透性过渡孔在疾病表型和药物筛选中的研究
线粒体作为Ca2+信号的“水槽”,线粒体Ca2+摄取将生理刺激与增加的ATP产生联系起来。然而,线粒体Ca2+超载可以通过打开线粒体通透性过渡孔(mPTP)诱导细胞灾难。这个孔是线粒体内膜上的一个大的传导途径,导致生物能量崩溃,似乎代表了许多疾病中细胞死亡的最终共同途径。mPTP作为疾病结局决定因素的作用在心脏、大脑和肾脏的缺血/再灌注损伤中得到了最好的证实,它也与神经退行性疾病和肌肉营养不良有关。由于孔隙打开的概率可以被药物调节,它代表了药物发现转化研究的一个有用的药理学靶点。在本单元中描述的是一种利用分离的线粒体来量化这种现象的方案,并为Ca2+失衡的表型筛选开发一个高通量平台。©2019作者。这是一篇基于知识共享署名许可协议的开放获取文章,该协议允许在任何媒体上使用、分发和复制,前提是正确引用原始作品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


