Asymmetric organocatalytic (3 + 2) annulation of propargylic alcohols with indolylnaphthalenols: synergistic construction of axial and central chirality†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d2qo01625g
引用次数: 0
Abstract
Organocatalytic enantioselective construction of chiral spiro N,N-acetal carbon stereocenters and axially chiral 3-arylindoles has been achieved via a chiral phosphoric acid (CPA)-catalyzed (3 + 2) annulation of α-(3-isoindolinonyl) propargylic alcohols with 1-(3-indolyl)naphthalen-2-ols, affording a broad scope of pyrrolo[1,2-a]indoles bearing both enantioenriched spiro isoindolinone-indoline and atropisomeric naphthalenol frameworks. Based on control experiments and our previous work, a possible mechanism was proposed accordingly.
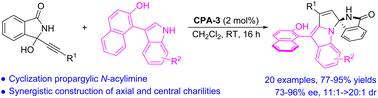
炔丙醇与吲哚酚的不对称有机催化(3+2)环化:轴向和中心手性的协同构建†
通过手性磷酸(CPA)催化的α-(3-异吲哚基)炔丙醇与1-(3-吲哚基)萘-2-醇的(3+2)环化反应,提供了广泛的吡咯并[1,2-a]吲哚,其具有对映体富集的螺-异吲哚啉酮-吲哚啉和阻聚萘酚骨架。基于控制实验和我们之前的工作,相应地提出了一种可能的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


