In vitro generation of tau aggregates conformationally distinct from parent tau seeds of Alzheimer's brain.
IF 1.6
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 9
Abstract
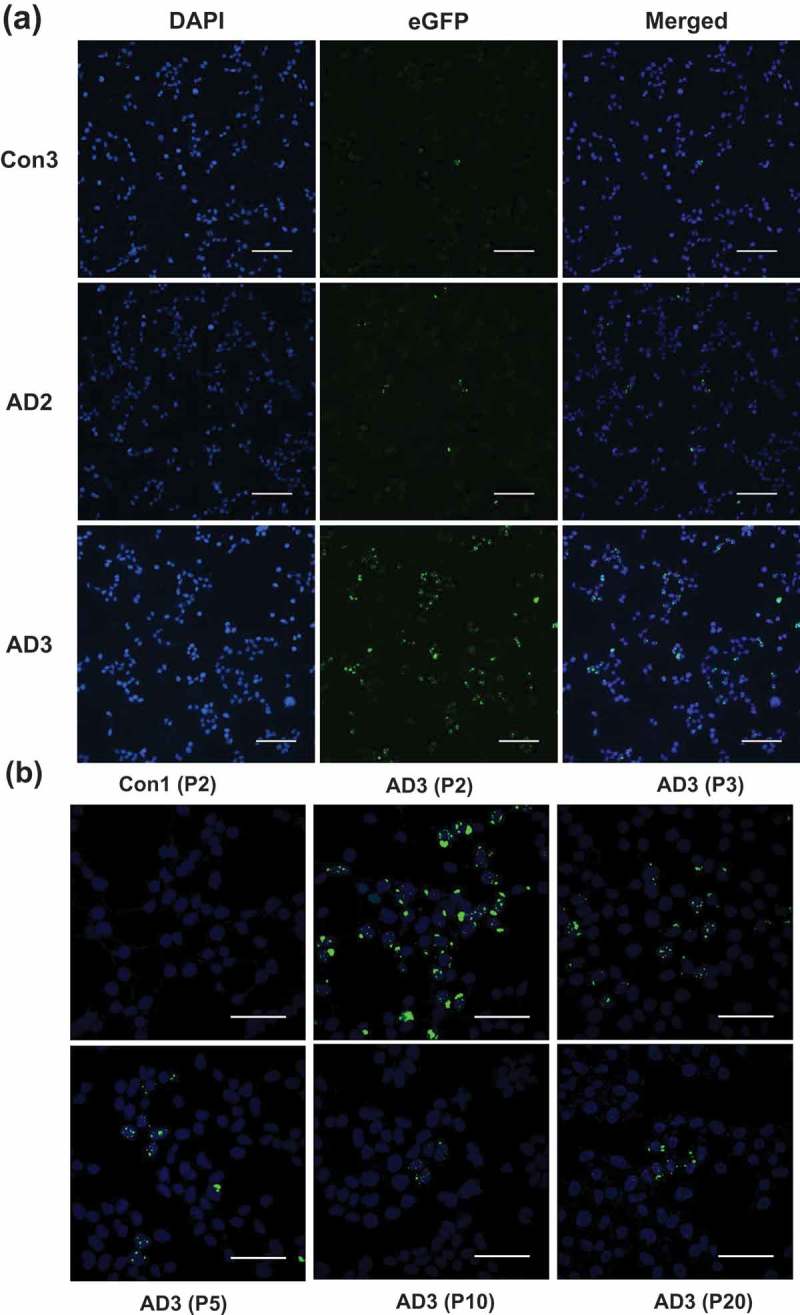
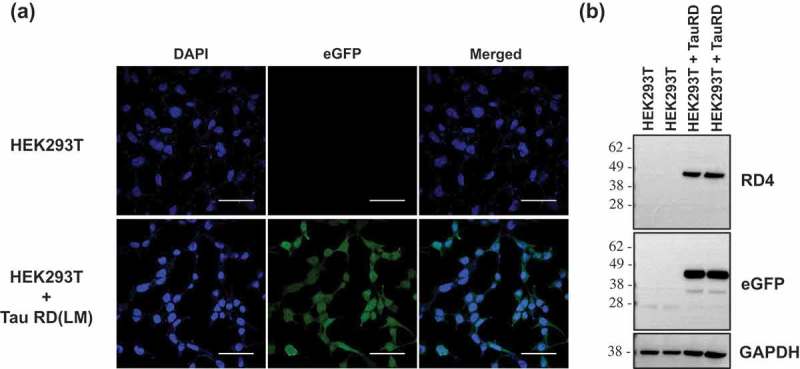
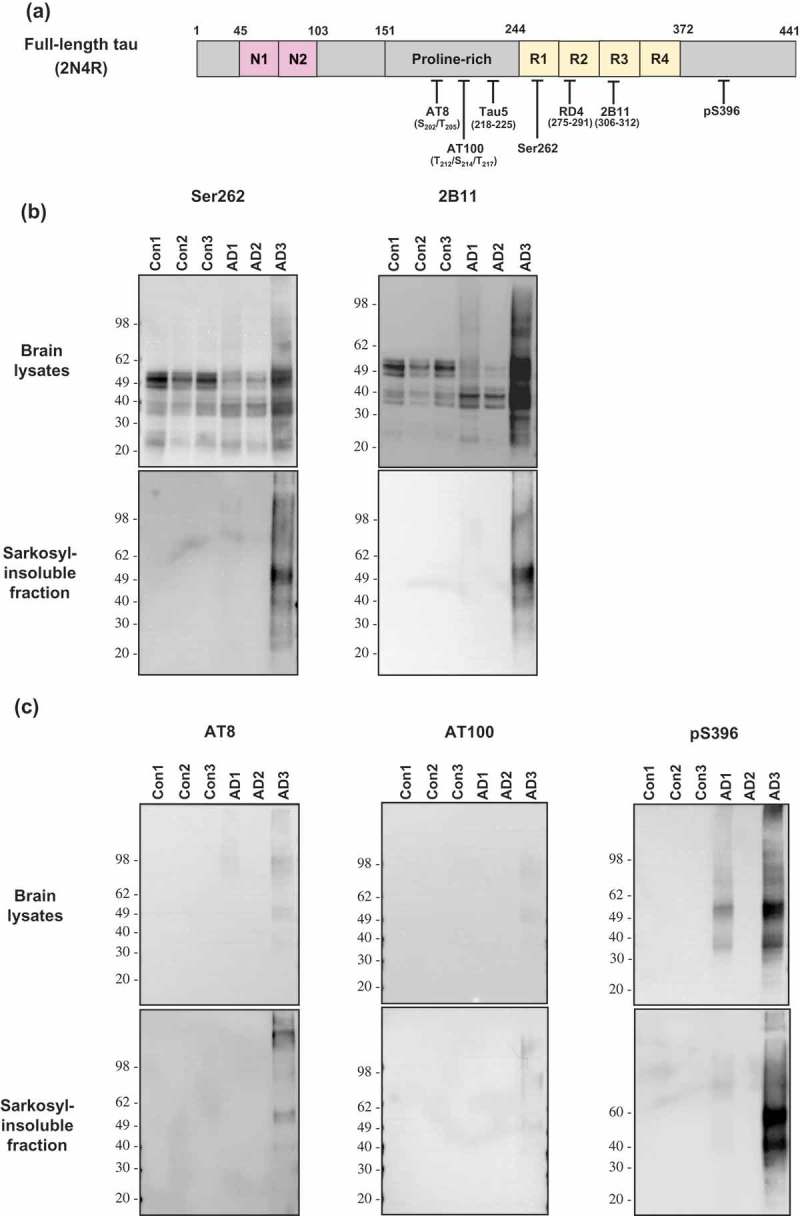
ABSTRACT Normal monomeric tau can be converted into pathogenic aggregates and acquire protease resistance in a prion-like manner. This acquisition of partial protease-resistance in tau aggregates has to date only been partially investigated in various studies exploring the prion-like properties of tau. In this study, we induced the aggregation of tau repeat domain (RD) in cultured cells using detergent insoluble fractions of Alzheimer’s brain tissue as seeds. The seeded aggregation of tau RD in cultured cells formed a ~7 kDa protease-resistant fragment in contrast to the ~12 kDa tau fragment characteristic of the AD seeds, suggesting that the in vitro generated tau aggregates were conformationally distinct from parent seeds.
体外生成的tau聚集体构象不同于阿尔茨海默病大脑的母体tau种子。
正常的单体tau蛋白可以转化为致病性聚集体,并以朊病毒样的方式获得蛋白酶抗性。迄今为止,在探索tau蛋白的朊病毒样特性的各种研究中,仅对tau蛋白聚集体中部分蛋白酶抗性的获得进行了部分研究。在这项研究中,我们使用阿尔茨海默氏症脑组织的洗涤剂不溶性部分作为种子,在培养细胞中诱导tau重复结构域(RD)的聚集。tau RD在培养细胞中的种子聚集形成了一个~7 kDa的蛋白酶抗性片段,而AD种子的特征是~12 kDa的tau片段,这表明体外产生的tau聚集物与亲本种子的构象不同。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Prion
生物-生化与分子生物学
CiteScore
5.20
自引率
4.30%
发文量
13
审稿时长
6-12 weeks
期刊介绍:
Prion is the first international peer-reviewed open access journal to focus exclusively on protein folding and misfolding, protein assembly disorders, protein-based and structural inheritance. The goal is to foster communication and rapid exchange of information through timely publication of important results using traditional as well as electronic formats. The overriding criteria for publication in Prion are originality, scientific merit and general interest.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


