Synthesis and Evaluation of Baylis-Hillman Reaction Derived Imidazole and Triazole Cinnamates as Antifungal Agents.
International Journal of Medicinal Chemistry
Pub Date : 2018-10-16
eCollection Date: 2018-01-01
DOI:10.1155/2018/5758076
引用次数: 1
Abstract
Allylic acetates derived from Baylis-Hillman reaction undergo efficient nucleophilic isomerization with imidazoles and triazoles to provide imidazolylmethyl and triazolylmethyl cinnamates stereoselectively. Antifungal evaluation of these derivatives against Cryptococcus neoformans exhibits good minimum inhibitory concentration values. These compounds exhibit low toxicity in proliferating MCF-7 breast cancer cell line. Structure activity relationship studies indicate that halogenated aromatic derivatives provide better antifungal activity.
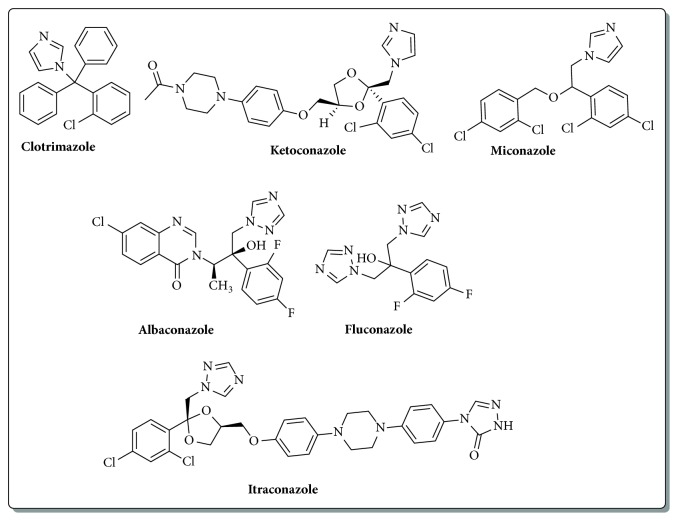
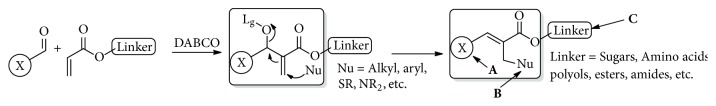
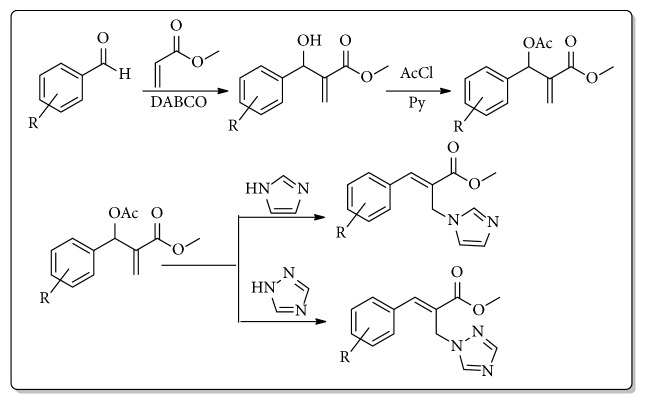
Baylis-Hillman反应衍生咪唑和三唑肉桂酸酯的合成与评价。
由Baylis-Hillman反应得到的烯丙酸酯与咪唑和三唑进行了高效的亲核异构化反应,得到立体选择性咪唑甲基肉桂酸酯和三唑甲基肉桂酸酯。这些衍生物对新型隐球菌的抗真菌评价显示出良好的最小抑制浓度值。这些化合物对增殖的MCF-7乳腺癌细胞系表现出低毒性。构效关系研究表明,卤代芳香衍生物具有较好的抗真菌活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Medicinal Chemistry
CHEMISTRY, MEDICINAL-
自引率
0.00%
发文量
0
期刊介绍:
International Journal of Medicinal Chemistry is a peer-reviewed, Open Access journal that publishes original research articles as well as review articles in all areas of chemistry associated with drug discovery, design, and synthesis. International Journal of Medicinal Chemistry is a peer-reviewed, Open Access journal that publishes original research articles as well as review articles in all areas of chemistry associated with drug discovery, design, and synthesis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


