Sylvie Janssens, Michael Schotsaert, Lara Manganaro, Marion Dejosez, Viviana Simon, Adolfo García-Sastre, Thomas P. Zwaka
下载PDF
{"title":"FACS-Mediated Isolation of Neuronal Cell Populations From Virus-Infected Human Embryonic Stem Cell–Derived Cerebral Organoid Cultures","authors":"Sylvie Janssens, Michael Schotsaert, Lara Manganaro, Marion Dejosez, Viviana Simon, Adolfo García-Sastre, Thomas P. Zwaka","doi":"10.1002/cpsc.65","DOIUrl":null,"url":null,"abstract":"<p>Organoids—or pluripotent stem cell–derived in vitro-grown simplified mini organs—have become a tremendously important model to study human organ development and disease. To restrict the noise inherent to the heterogeneous cell mixtures derived from organoid cultures, we developed a new technique of fluorescence-assisted cell sorting (FACS) of virus-infected cerebral organoid cultures. This method still includes the advantage of growing cells in a more natural environment than traditional cell culture, but now renders samples suitable for downstream cell type-specific multi-omics analyses. The protocol starts from stem cell-derived mature brain organoids and includes steps for: preparing the culture for viral infection, production of the viral stocks, FACS sample preparation, and gating and sorting implementation. The protocol has been developed for Zika virus infection, but can be extrapolated to other viruses or fluorescent marker expression as illustrated in an alternate protocol using a single-cycle lentivirus expressing a fluorescent reporter protein. © 2018 by John Wiley & Sons, Inc.</p>","PeriodicalId":53703,"journal":{"name":"Current Protocols in Stem Cell Biology","volume":"48 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2018-10-24","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpsc.65","citationCount":"14","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols in Stem Cell Biology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpsc.65","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"Biochemistry, Genetics and Molecular Biology","Score":null,"Total":0}
引用次数: 14
引用
批量引用
Abstract
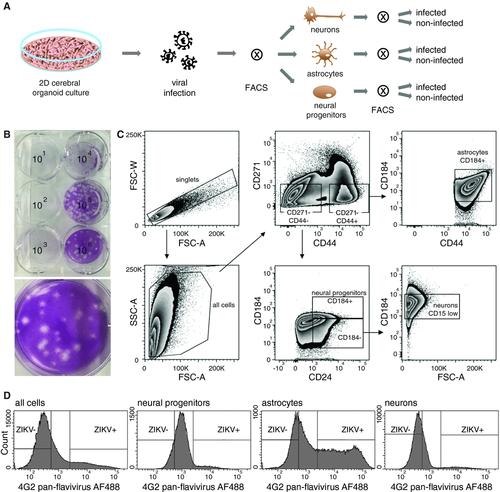
Organoids—or pluripotent stem cell–derived in vitro-grown simplified mini organs—have become a tremendously important model to study human organ development and disease. To restrict the noise inherent to the heterogeneous cell mixtures derived from organoid cultures, we developed a new technique of fluorescence-assisted cell sorting (FACS) of virus-infected cerebral organoid cultures. This method still includes the advantage of growing cells in a more natural environment than traditional cell culture, but now renders samples suitable for downstream cell type-specific multi-omics analyses. The protocol starts from stem cell-derived mature brain organoids and includes steps for: preparing the culture for viral infection, production of the viral stocks, FACS sample preparation, and gating and sorting implementation. The protocol has been developed for Zika virus infection, but can be extrapolated to other viruses or fluorescent marker expression as illustrated in an alternate protocol using a single-cycle lentivirus expressing a fluorescent reporter protein. © 2018 by John Wiley & Sons, Inc.
facs介导的从病毒感染的人胚胎干细胞衍生的脑类器官培养中分离神经细胞群的研究
类器官,即体外培养的简化微型器官衍生的多能干细胞,已经成为研究人体器官发育和疾病的一个非常重要的模型。为了限制来自类器官培养的异质细胞混合物所固有的噪声,我们开发了一种新的病毒感染脑类器官培养的荧光辅助细胞分选(FACS)技术。这种方法仍然包括在比传统细胞培养更自然的环境中培养细胞的优势,但现在使样品适合下游细胞类型特异性多组学分析。该方案从干细胞衍生的成熟脑类器官开始,包括以下步骤:准备病毒感染培养,病毒库存的生产,FACS样品制备,以及门控和分选实施。该方案是针对寨卡病毒感染制定的,但也可推广到其他病毒或荧光标记物表达,如使用表达荧光报告蛋白的单周期慢病毒的替代方案所示。©2018 by John Wiley &儿子,Inc。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


