Expression, characterization and one step purification of heterologous glucose oxidase gene from Aspergillus niger ATCC 9029 in Pichia pastoris
Q4 Biochemistry, Genetics and Molecular Biology
引用次数: 13
Abstract
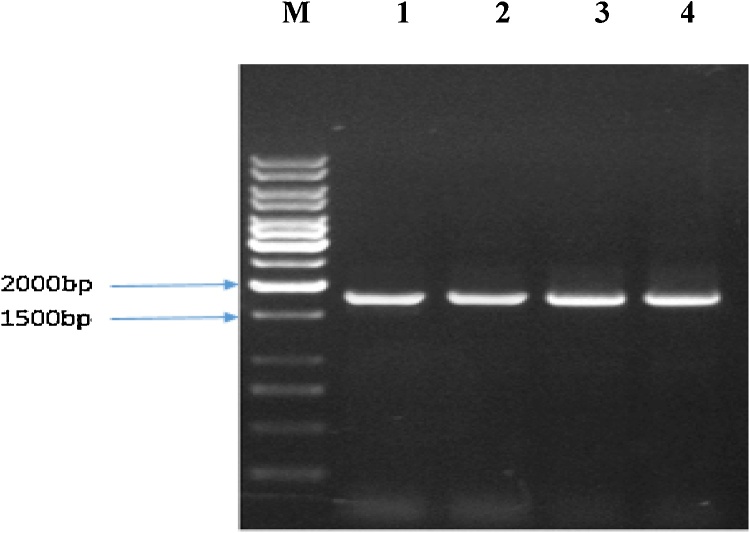
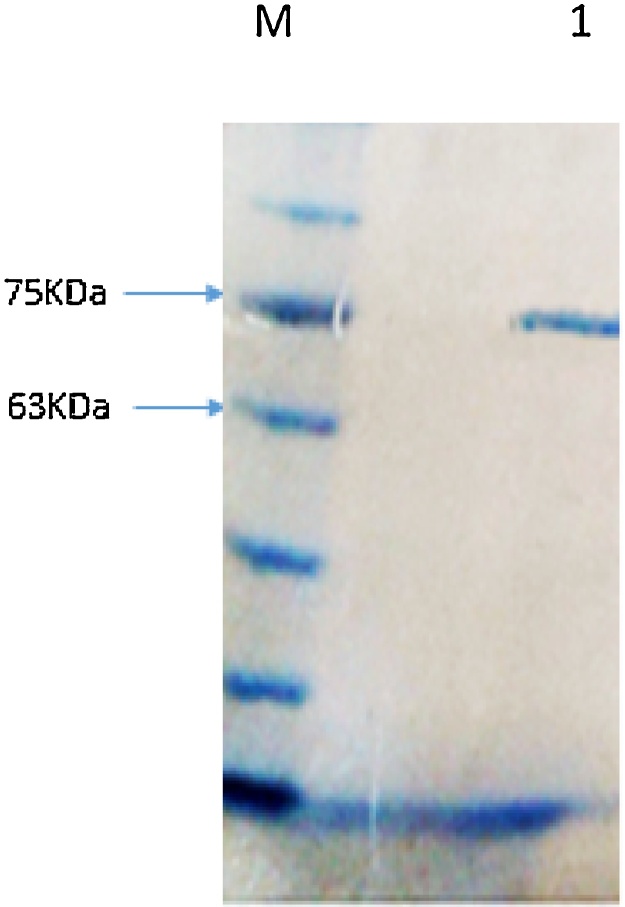
Glucose Oxidase (GOD), is a common flavoprotein from Aspergillus niger ATCC 9029 with a broad application in biotechnology, food and medical industries. In this study, GOD gene was cloned into the expression vector, pPIC9 and screened by the alcohol oxidase promoter. The enzyme production increased at 28 °C. GOD activity induced by 1.0% methanol and the highest level of GOD production was the result of shaking rate at 225 rpm. The highest enzyme activity obtained at a pH value ranged from 5 to 7 at 50 °C. The enzyme was stable at a broad pH range and temperature.

毕赤酵母中黑曲霉ATCC 9029异源葡萄糖氧化酶基因的表达、鉴定及一步纯化
葡萄糖氧化酶(GOD)是黑曲霉ATCC 9029中常见的黄酮类蛋白,在生物技术、食品和医疗等领域有着广泛的应用。本研究将GOD基因克隆到表达载体pPIC9中,通过乙醇氧化酶启动子进行筛选。在28 °C时酶产量增加。1.0%甲醇诱导的GOD活性和最高的GOD产量是在225 rpm的转速下产生的。在50 °C条件下,pH值为5 ~ 7时酶活性最高。该酶在较宽的pH范围和温度下稳定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

EuPA Open Proteomics
Biochemistry, Genetics and Molecular Biology-Biochemistry
自引率
0.00%
发文量
0
审稿时长
103 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


