Iodine(iii)-mediated dehydroaromatization of cyclohexanones with primary amines and CD3SSO3Na to access ortho-SCD3 anilines†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00481c
引用次数: 0
Abstract
Iodine(iii)-mediated dehydroaromatization of cyclohexanones with primary amines and CD3SSO3Na has been developed, providing direct access to ortho-SCD3 anilines with the formation of C–N and C–S bonds. Detailed mechanism studies indicate that hypervalent iodine(iii) plays dual roles as an efficient mediator and an oxidant, and the continuously generated α-acetoxylated ketones and α-SCD3 ketones are the key intermediates in the current three-component reactions. The present transformations demonstrate excellent chemo-selectivity, and only employ an iodine(iii) reagent as a stoichiometric mediator, making the strategy applicable for late-stage modification of numerous pharmaceuticals.
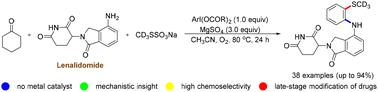
碘(iii)介导的环己酮与伯胺和CD3SSO3Na的脱氢芳构化以获得邻位-SCD3苯胺†
碘(iii)介导的环己酮与伯胺和CD3SSO3Na的脱氢芳构化已经开发出来,通过形成C–N和C–S键提供了直接接触邻位-SCD3苯胺的途径。详细的机理研究表明,高价碘(iii)具有高效介质和氧化剂的双重作用,连续生成的α-乙酰氧基化酮和α-SCD3酮是当前三组分反应的关键中间体。目前的转化显示出优异的化学选择性,并且仅使用碘(iii)试剂作为化学计量介质,使得该策略适用于许多药物的后期修饰。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


