An alternative metal-free amination approach to 3-trifluoromethyl aniline derivatives: the major products under Kröhnke pyridine synthesis conditions†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d3qo00493g
引用次数: 0
Abstract
As an alternative metal-free amination, we report a simple and efficient annulation reaction of 1-(3,3,3-trifluoro-2-oxopropyl)pyridin-1-ium bromide and α,β-unsaturated carbonyl compounds with NH4OAc or amines. With the developed protocol, a series of 3-trifluoromethyl aniline derivatives as the major products were obtained in good to excellent yields under Kröhnke pyridine synthesis conditions. The reaction proceeds via a cascade process involving the 1,4-Michael addition of the pyridinium ylide to an α,β-unsaturated carbonyl compound, followed by intramolecular addition of a carbanion to the keto carbonyl group to form a dienone intermediate.
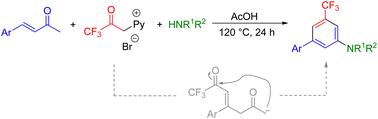
3-三氟甲基苯胺衍生物的另一种无金属胺化方法:Kröhnke吡啶合成条件下的主要产物†
作为一种替代的无金属胺化反应,我们报道了1-(3,3,3-三氟-2-氧丙基)吡啶-1-溴化铵和α,β-不饱和羰基化合物与NH4OAc或胺的简单有效的环化反应。根据所开发的方案,在Kröhnke吡啶合成条件下,以良好至优异的产率获得了一系列3-三氟甲基苯胺衍生物作为主要产物。反应通过级联过程进行,包括吡啶鎓叶立德与α,β-不饱和羰基化合物的1,4-Michel加成,然后碳阴离子与酮羰基的分子内加成,形成二烯酮中间体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


