Asymmetric synthesis of 7-membered-ring-bridged 3,4-fused tricyclic indoles via Friedel–Crafts alkylation/annulation†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2023-01-01
DOI:10.1039/d2qo01982e
引用次数: 0
Abstract
With N-methyl-4-aminoindole as the 1,4-bisnucleophile, the regio- and enantioselective Friedel–Crafts alkylation/N-hemiacetalization and dehydration sequence with β,γ-unsaturated α-ketoesters was reported using 5 mol% Ni(ii)-PyBPI complex, affording diverse chiral 7-membered-ring-bridged 3,4-fused tricyclic indoles in good results (up to 91% yield and 97% ee). The by-products were 6-membered-ring-bridged 4,5-fused tricyclic indoles.
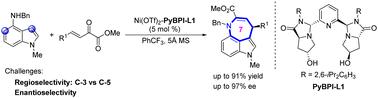
通过Friedel-Crafts烷基化/环化不对称合成7-元环桥3,4-氟三环吲哚†
以N-甲基-4-氨基吲哚为1,4-双亲核试剂,用5mol%Ni(ii)-PyBPI配合物报道了与β,γ-不饱和α-酮酯的区域和对映选择性Friedel–Crafts烷基化/N-半缩醛化和脱水序列,得到了各种手性的7-元环桥3,4-氟三环吲哚,结果良好(产率高达91%,ee高达97%)。副产物是6-元环桥4,5-氟三环吲哚。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


