Lysine acetylation of major Chlamydia trachomatis antigens
Q4 Biochemistry, Genetics and Molecular Biology
引用次数: 3
Abstract
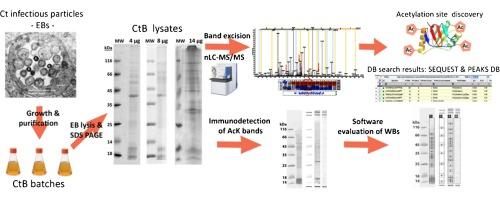
Chlamydia trachomatis (Ct) is a human pathogen causing trachoma and infertility. We investigated acetylation at lysine residues of chlamydial antigenic proteins: major outer membrane protein (MOMP), 60 kDa chaperonin (chlamydial Hsp60), elongation factor G (EF-G), enolase and the polymorphic membrane proteins PmpB, PmpE and PmpF. 60 kDa chaperonin, EF-G and PmpB showed the highest degree of acetylation.
Our data show that important Ct antigens could be post-translationally modified by acetylation of lysine residues at multiple sites. Further studies are needed to investigate total acetylome of Ct and the impact PTMs might have on Ct biology and pathogenicity.
沙眼衣原体主要抗原赖氨酸乙酰化
沙眼衣原体(Ct)是一种引起沙眼和不孕的人类病原体。我们研究了衣原体抗原蛋白赖氨酸残基的乙酰化:主要外膜蛋白(MOMP)、60 kDa伴蛋白(衣原体Hsp60)、延伸因子G (EF-G)、烯醇酶和多态膜蛋白PmpB、PmpE和PmpF。60 kDa的伴侣蛋白、EF-G和PmpB乙酰化程度最高。我们的数据表明,重要的Ct抗原可以通过赖氨酸残基在多个位点的乙酰化进行翻译后修饰。Ct的总乙酰化程度以及PTMs对Ct生物学和致病性的影响有待进一步研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

EuPA Open Proteomics
Biochemistry, Genetics and Molecular Biology-Biochemistry
自引率
0.00%
发文量
0
审稿时长
103 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


