Intestinal Permeability of Artesunate-Loaded Solid Lipid Nanoparticles Using the Everted Gut Method.
Journal of drug delivery
Pub Date : 2018-04-30
eCollection Date: 2018-01-01
DOI:10.1155/2018/3021738
引用次数: 28
Abstract
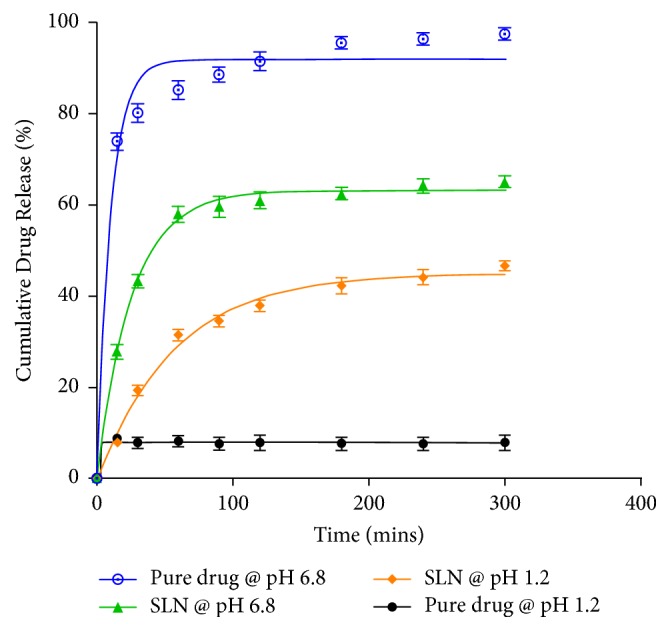
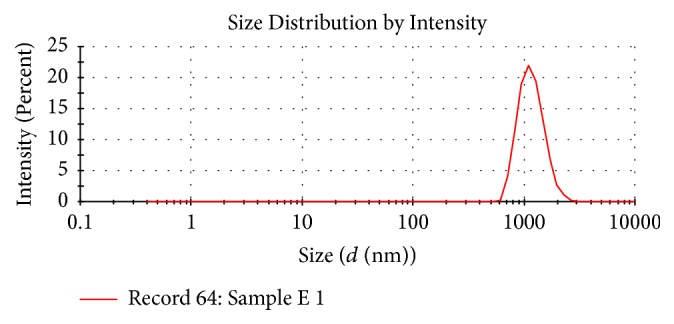
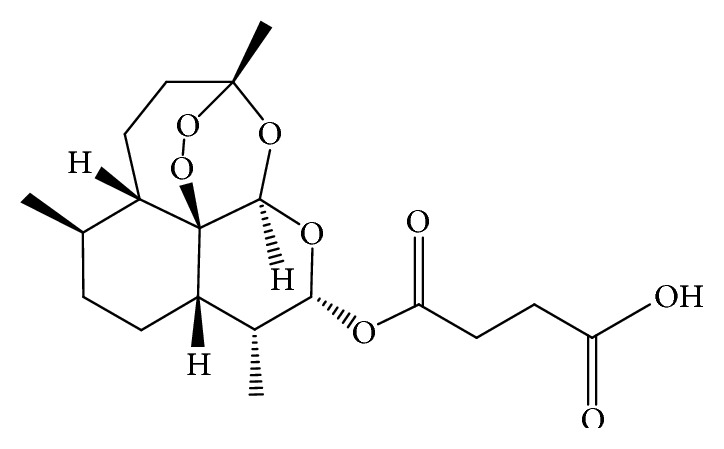
Background Artesunate is one of the most potent, rapidly acting and therapeutically versatile antimalarial drugs. Its efficacy is hampered by poor aqueous solubility and stability resulting in low oral bioavailability. Recent efforts to nanoformulate artesunate have shown great potential of improving its dissolution profile and bioavailability. However, no study has yet been done to investigate the intestinal permeability of these nanoformulations, which is a critical determinant of systemic absorption. Objective of the Study The main aim of the study was to determine the intestinal permeability of artesunate-loaded solid lipid nanoparticles (SLN). Method The microemulsion dilution technique was used to fabricate artesunate-loaded solid lipid nanoparticles. In vitro drug release studies were performed at pH 1.2 and 6.8 using the dialysis membrane method. The everted gut sac method was used to assess the intestinal permeability of the prepared nanoparticles. Results The average particle size was 1109 nm and the polydispersity index (PDI) was 0.082. The zeta potential was found to be −20.7 mV. The encapsulation efficiency of the solid lipid nanoparticles obtained was 51.7%. At both pH 1.2 and 6.8, pure artesunate was rapidly released within the first 30 mins while the SLN showed a biphasic release pattern with an initial burst release during the first hour followed by a prolonged release over time. The rate of drug release increased with increasing pH. The apparent permeability (Papp) of SLN was found to be greater (0.169 mg/cm2) as compared to that of pure artesunate (0.117 mg/cm2) at the end of the experiment. Conclusion The results obtained in this study showed that the microemulsion dilution technique can be used to formulate artesunate solid lipid nanoparticles. The formulation exhibited a sustained drug release profile. The intestinal permeability of artesunate could be enhanced by the nanoformulation.
利用肠外法研究含青蒿琥酯的固体脂质纳米颗粒的肠通透性。
背景:青蒿琥酯是最有效、作用最迅速和治疗用途最广泛的抗疟疾药物之一。其水溶性和稳定性差,导致口服生物利用度低,影响了其疗效。近年来对青蒿琥酯纳米配方的研究表明,在提高其溶出度和生物利用度方面具有很大的潜力。然而,目前还没有研究调查这些纳米制剂的肠通透性,这是系统吸收的关键决定因素。研究目的:本研究的主要目的是测定载青蒿琥酯固体脂质纳米颗粒(SLN)的肠通透性。方法:采用微乳液稀释法制备青蒿琥酯载固体脂质纳米颗粒。采用透析膜法在pH 1.2和6.8下进行体外药物释放研究。采用肠囊外翻法评价制备的纳米颗粒的肠通透性。结果:平均粒径为1109 nm, PDI为0.082。zeta电位为-20.7 mV。制备的固体脂质纳米颗粒包封率为51.7%。在pH为1.2和6.8时,纯青蒿琥酯在前30分钟内迅速释放,而SLN则表现为双相释放模式,最初在第一个小时内释放,随后随着时间的推移释放时间延长。实验结束时,SLN的表观渗透性(Papp) (0.169 mg/cm2)高于纯青蒿琥酯(0.117 mg/cm2)。结论:本研究结果表明,微乳稀释技术可用于制备青蒿琥酯固体脂质纳米颗粒。该制剂具有持久的药物释放特性。纳米制剂可提高青蒿琥酯的肠通透性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


