Benzil/triethylamine: a photo-reducing system for Cu2.
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 2
Abstract
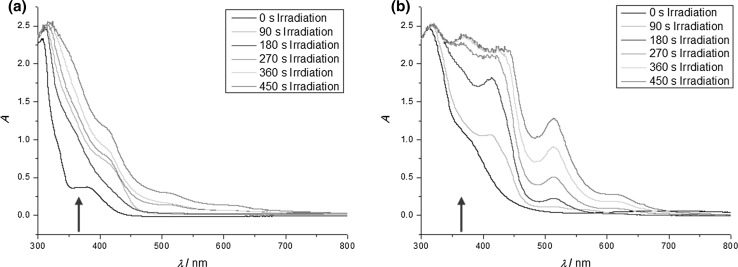
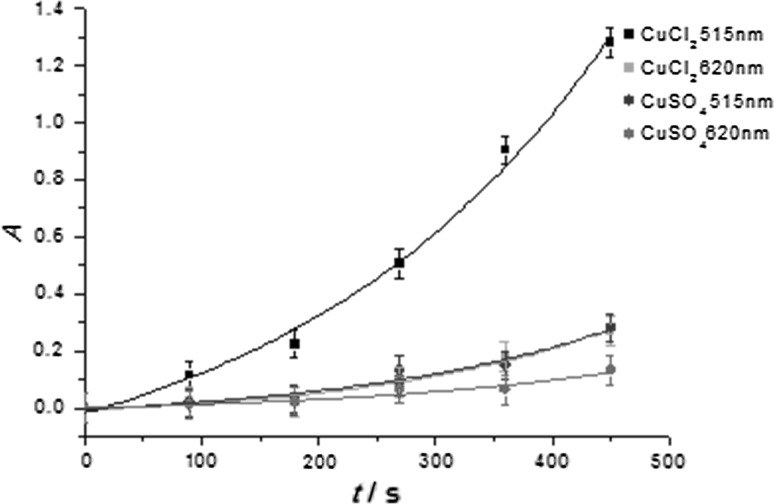
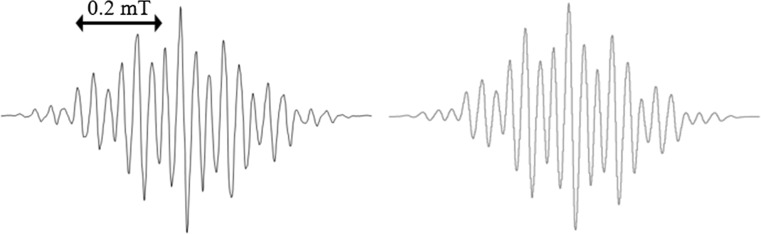
Abstract: We have investigated the photo-induced reduction of Cu2+-Cu0 using benzil/triethylamine mixtures. The formation of elemental Cu is indicated by the appearance of its characteristic plasmon absorption peaks at 515 nm and 620 nm. Importantly, the nature of the counterion of the Cu2+ salt affects the reduction process. In the presence of Cl-, the reduction proceeds faster than with SO42-. Photo-induced electron transfer between excited benzil and triethylamine leads to the benzil radical anion, which acts as the reducing agent for Cu2+ and generates Cu0.
Graphical abstract:
苄基/三乙胺:光还原Cu2体系。
摘要:本文研究了苯基/三乙胺混合物光诱导还原Cu2+-Cu0的反应。元素铜在515 nm和620 nm处的特征等离激元吸收峰表明了元素铜的形成。重要的是,Cu2+盐的反离子性质影响了还原过程。Cl-存在时,还原速度比SO42-快。受激发的苄基与三乙胺之间发生光致电子转移,产生苄基阴离子,作为Cu2+的还原剂生成Cu0。图形化的简介:
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Monatshefte Fur Chemie
化学-化学综合
CiteScore
3.70
自引率
5.60%
发文量
116
审稿时长
3.3 months
期刊介绍:
"Monatshefte für Chemie/Chemical Monthly" was originally conceived as an Austrian journal of chemistry. It has evolved into an international journal covering all branches of chemistry. Featuring the most recent advances in research in analytical chemistry, biochemistry, inorganic, medicinal, organic, physical, structural, and theoretical chemistry, Chemical Monthly publishes refereed original papers and a section entitled "Short Communications". Reviews, symposia in print, and issues devoted to special fields will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


