Protein chainmail variants in dsDNA viruses.
IF 1
Q4 BIOPHYSICS
引用次数: 8
Abstract
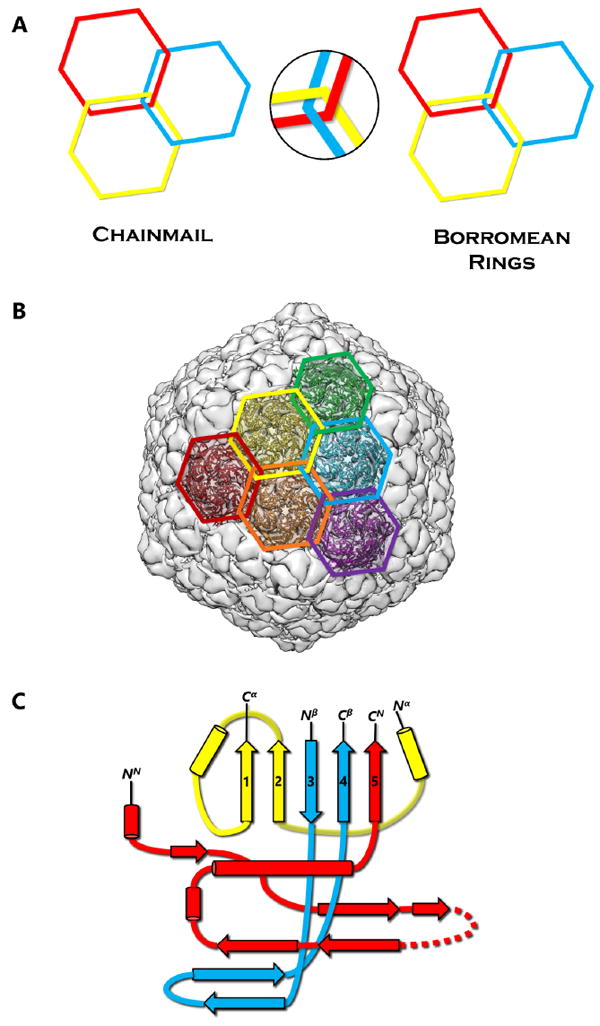
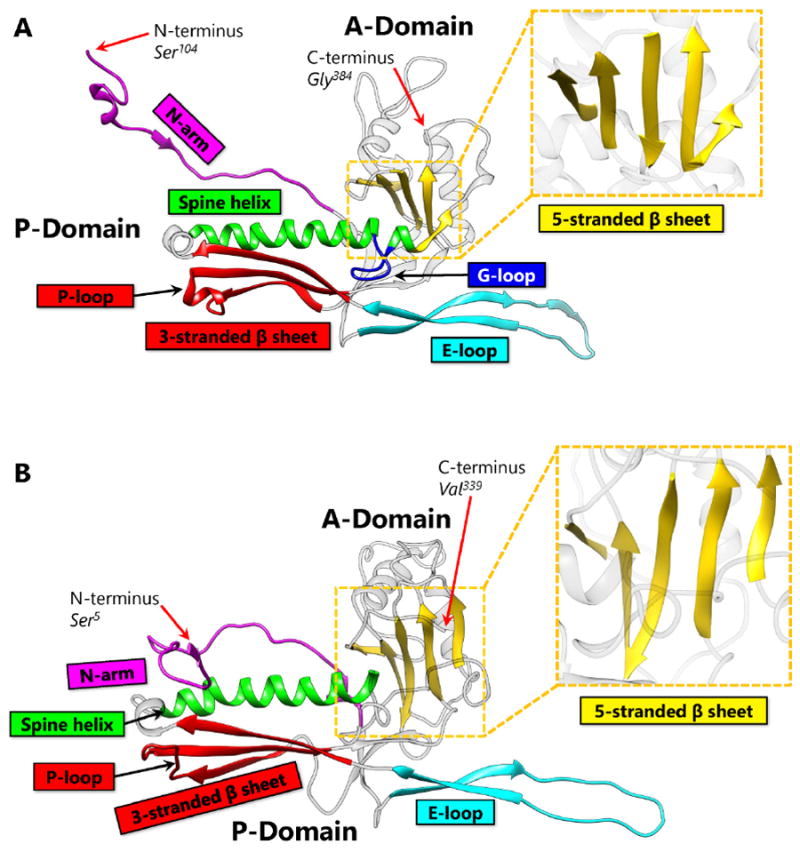
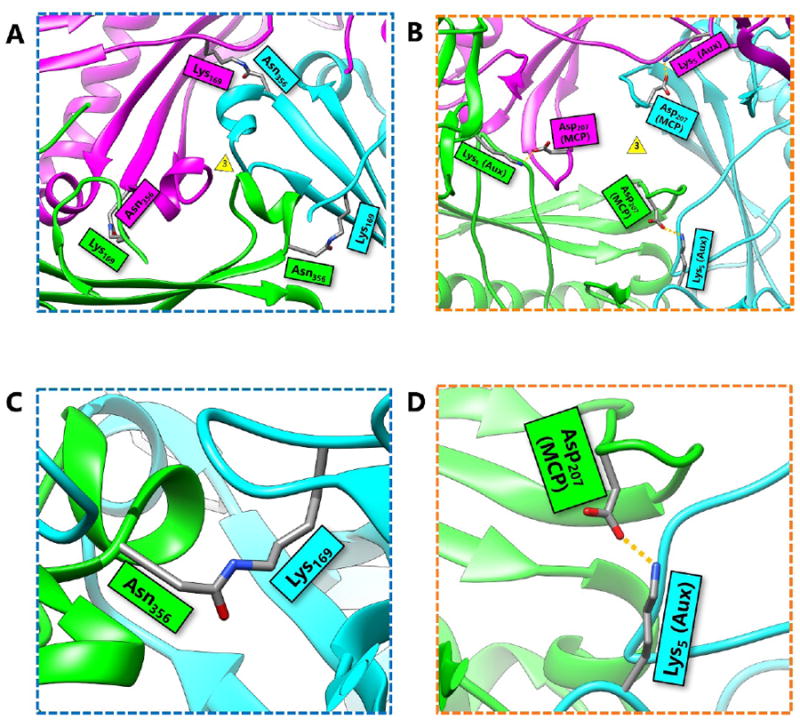
First discovered in bacteriophage HK97, biological chainmail is a highly stable system formed by concatenated protein rings. Each subunit of the ring contains the HK97-like fold, which is characterized by its submarine-like shape with a 5-stranded β sheet in the axial (A) domain, spine helix in the peripheral (P) domain, and an extended (E) loop. HK97 capsid consists of covalently-linked copies of just one HK97-like fold protein and represents the most effective strategy to form highly stable chainmail needed for dsDNA genome encapsidation. Recently, near-atomic resolution structures enabled by cryo electron microscopy (cryoEM) have revealed a range of other, more complex variants of this strategy for constructing dsDNA viruses. The first strategy, exemplified by P22-like phages, is the attachment of an insertional (I) domain to the core 5-stranded β sheet of the HK97-like fold. The atomic models of the Bordetella phage BPP-1 showcases an alternative topology of the classic HK97 topology of the HK97-like fold, as well as the second strategy for constructing stable capsids, where an auxiliary jellyroll protein dimer serves to cement the non-covalent chainmail formed by capsid protein subunits. The third strategy, found in lambda-like phages, uses auxiliary protein trimers to stabilize the underlying non-covalent chainmail near the 3-fold axis. Herpesviruses represent highly complex viruses that use a combination of these strategies, resulting in four-level hierarchical organization including a non-covalent chainmail formed by the HK97-like fold domain found in the floor region. A thorough understanding of these structures should help unlock the enigma of the emergence and evolution of dsDNA viruses and inform bioengineering efforts based on these viruses.
dsDNA病毒中的蛋白质链甲变异。
首先在噬菌体HK97中发现,生物链甲是由连接的蛋白质环形成的高度稳定的系统。环的每个亚基包含hk97样褶皱,其特征是其呈海底状,轴向(a)结构域为5链β片,外周(P)结构域为棘螺旋,延伸(E)环。HK97衣壳仅由一个HK97样折叠蛋白的共价连锁拷贝组成,代表了形成dsDNA基因组封装所需的高度稳定链甲的最有效策略。最近,通过低温电子显微镜(cryoEM)实现的近原子分辨率结构揭示了构建dsDNA病毒策略的一系列其他更复杂的变体。以p22样噬菌体为例,第一种策略是将插入(I)结构域附着在hk97样折叠的核心5链β片上。博德特拉噬菌体BPP-1的原子模型展示了HK97样折叠的经典HK97拓扑的另一种拓扑结构,以及构建稳定衣壳的第二种策略,其中辅助jellyroll蛋白二聚体用于巩固由衣壳蛋白亚基形成的非共价链甲。第三种策略是在类lambda噬菌体中发现的,它使用辅助蛋白三聚体来稳定3倍轴附近潜在的非共价链甲。疱疹病毒代表了高度复杂的病毒,它使用这些策略的组合,导致四层层次组织,包括由底区发现的hk97样折叠结构域形成的非共价链甲。对这些结构的透彻理解将有助于解开dsDNA病毒出现和进化的谜团,并为基于这些病毒的生物工程工作提供信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

AIMS Biophysics
BIOPHYSICS-
CiteScore
2.40
自引率
20.00%
发文量
16
审稿时长
8 weeks
期刊介绍:
AIMS Biophysics is an international Open Access journal devoted to publishing peer-reviewed, high quality, original papers in the field of biophysics. We publish the following article types: original research articles, reviews, editorials, letters, and conference reports. AIMS Biophysics welcomes, but not limited to, the papers from the following topics: · Structural biology · Biophysical technology · Bioenergetics · Membrane biophysics · Cellular Biophysics · Electrophysiology · Neuro-Biophysics · Biomechanics · Systems biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


