Unexpected course of reaction between (E)-2-aryl-1-cyano-1-nitroethenes and diazafluorene: why is there no 1,3-dipolar cycloaddition?
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
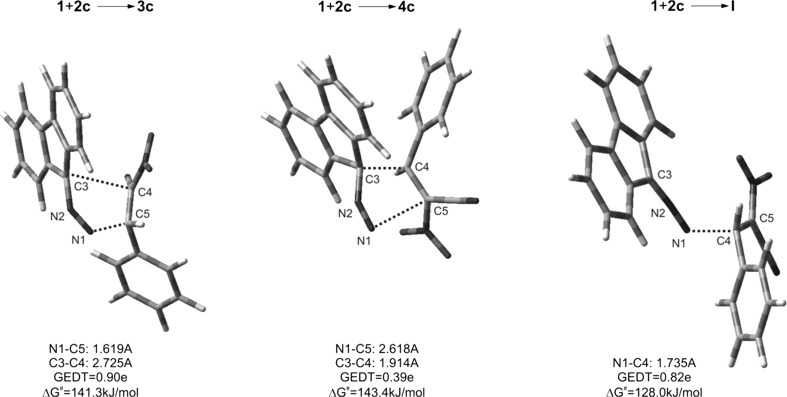
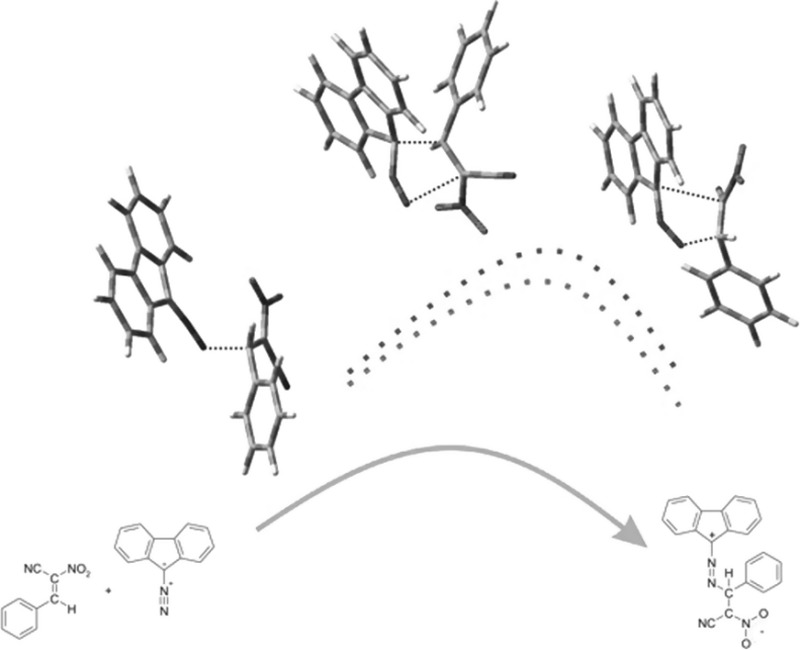
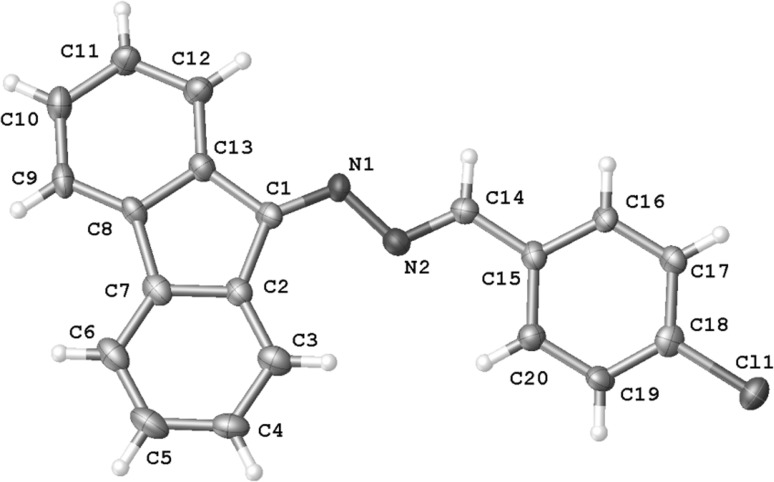
Abstract: Reactions between (E)-2-aryl-1-cyano-1-nitroethenes and diazafluorene lead to acyclic 2,3-diazabuta-1,3-diene derivatives, instead of the expected pyrazoline systems. DFT calculations suggest that this is a consequence of formation of zwitterionic structure in the first stage of the reaction. It must be noted that this is a specific property of the (E)-2-aryl-1-cyano-1-nitroethenes group, in contrast to most other conjugated nitroalkenes.
Graphical abstract:
(E)-2-芳基-1-氰基-1-硝基乙烯与二氮杂芴的意外反应过程:为什么没有 1,3-二极环化反应?
摘要:(E)-2-芳基-1-氰基-1-硝基乙烯与重氮芴的反应会产生无环的 2,3-二氮杂吲哚-1,3-二烯衍生物,而不是预期的吡唑啉体系。DFT 计算表明,这是反应第一阶段形成齐聚物结构的结果。必须指出的是,这是(E)-2-芳基-1-氰基-1-硝基乙烯基团的特殊性质,与大多数其他共轭硝基烯不同:
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Monatshefte Fur Chemie
化学-化学综合
CiteScore
3.70
自引率
5.60%
发文量
116
审稿时长
3.3 months
期刊介绍:
"Monatshefte für Chemie/Chemical Monthly" was originally conceived as an Austrian journal of chemistry. It has evolved into an international journal covering all branches of chemistry. Featuring the most recent advances in research in analytical chemistry, biochemistry, inorganic, medicinal, organic, physical, structural, and theoretical chemistry, Chemical Monthly publishes refereed original papers and a section entitled "Short Communications". Reviews, symposia in print, and issues devoted to special fields will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


