Cocaine Self-Administration Elevates GluN2B within dmPFC Mediating Heightened Cue-Elicited Operant Responding.
引用次数: 12
Abstract
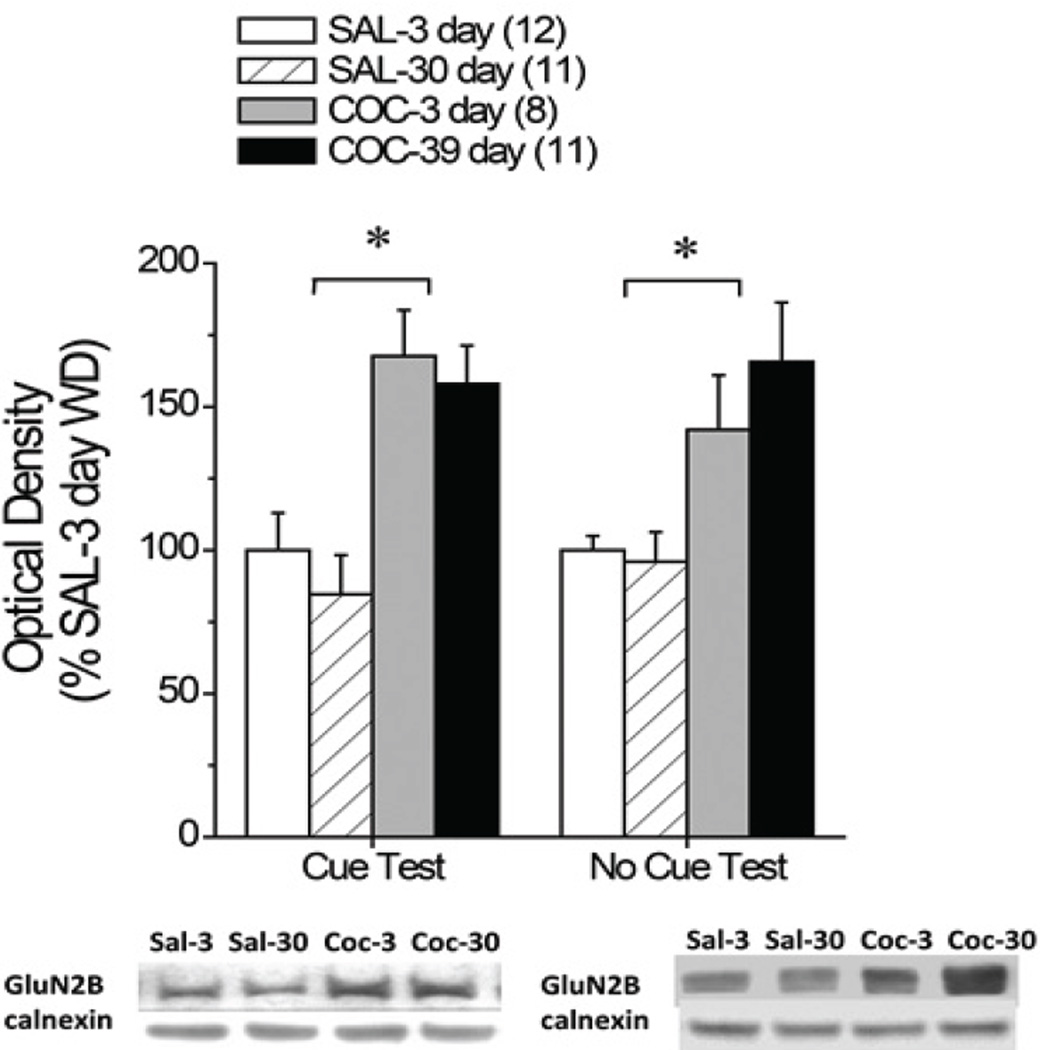
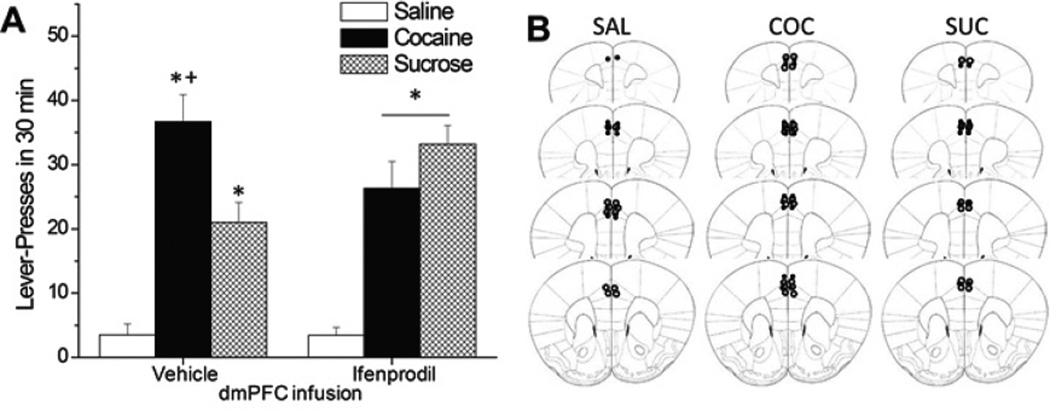
Cue-elicited drug-craving correlates with hyperactivity within prefrontal cortex (PFC), which is theorized to result from dysregulated excitatory neurotransmission. The NMDA glutamate receptor is highly implicated in addiction-related neuroplasticity. As NMDA receptor function is regulated critically by its GluN2 subunits, herein, we assayed the relation between incubated cue-elicited cocaine-seeking following extended access to intravenous cocaine (6 h/d; 0.25 mg/infusion for 10 d) and the expression of GluN2A/B receptor subunits within PFC sub regions during early versus late withdrawal (respectively, 3 vs. 30 days). Cocaine-seeking rats exhibited elevated GluN2B expression within the dorsomedial aspect of the PFC (dmPFC); this effect was apparent at both 3 and 30 days withdrawal and occurred in cocaine-experienced rats, regardless of experiencing an extinction test or not. Thus, elevated dmPFC GluN2B expression appears to reflect a pharmacodynamic response to excessive cocaine intake that is independent of the duration of drug withdrawal or re-exposure to drug-taking context. The functional relevance of elevated dmPFC GluN2B expression for drug-seeking was assessed by the local infusion of the prototypical GluN2B-selective antagonist ifenprodil (1.0 µg/side). Ifenprodil did not alter cue-elicited responding in animals with a history of saline self-administration. In contrast, ifenprodil lowered cue-elicited cocaine-seeking, while potentiating cue-elicited sucrose-seeking. Thus, the effects of an intra-dmPFC ifenprodil infusion upon cue reactivity are reinforcer-specific, arguing in favor of targeting GluN2B-containing NMDA receptors as a pharmacological strategy for reducing behavioral reactivity to drug-associated cues with the potential benefit of heightening the reinforcing properties of cues associated with non-drug primary rewards.
可卡因自我给药可提高dmPFC内GluN2B介导线索引发的高操作性反应。
线索引发的药物渴求与前额叶皮层(PFC)的过度活跃有关,这是由兴奋性神经传递失调引起的。NMDA谷氨酸受体与成瘾相关的神经可塑性密切相关。由于NMDA受体的功能受到GluN2亚基的关键调控,因此,我们分析了长时间静脉注射可卡因(6小时/天;0.25 mg/输注10 d),以及早期和晚期停药期间PFC亚区GluN2A/B受体亚单位的表达(分别为3天和30天)。可卡因寻求大鼠在PFC背内侧(dmPFC)表现出GluN2B表达升高;这种效果在戒断3天和30天都很明显,并且发生在有可卡因经验的大鼠身上,无论是否经历过灭绝试验。因此,升高的dmPFC GluN2B表达似乎反映了过量可卡因摄入的药效学反应,与停药时间或再次暴露于吸毒环境无关。通过局部输注典型GluN2B选择性拮抗剂伊芬普罗地尔(1.0µg/侧)来评估dmPFC GluN2B表达升高与药物寻找的功能相关性。在有生理盐水自我给药史的动物中,伊芬普罗地尔没有改变提示引起的反应。相比之下,伊芬地尔降低了线索引发的可卡因寻找,同时增强了线索引发的蔗糖寻找。因此,dmpfc内输注非芬普罗地尔对线索反应性的影响是强化物特异性的,这表明靶向glun2b - NMDA受体是一种降低对药物相关线索的行为反应性的药理学策略,并可能增强与非药物主要奖励相关的线索的强化特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


