Pharmacokinetic, Pharmacodynamic, and Safety Profile of a Novel Anti-CD28 Domain Antibody Antagonist in Healthy Subjects.
IF 2.3
4区 医学
Q3 PHARMACOLOGY & PHARMACY
引用次数: 27
Abstract
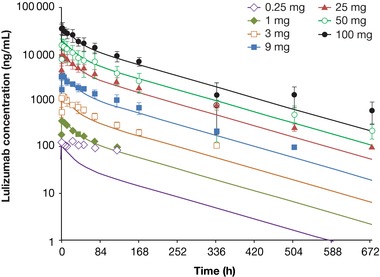
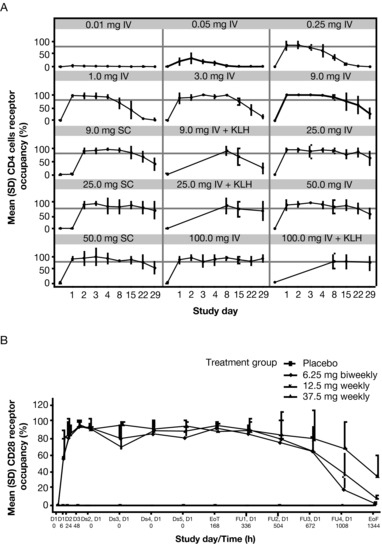
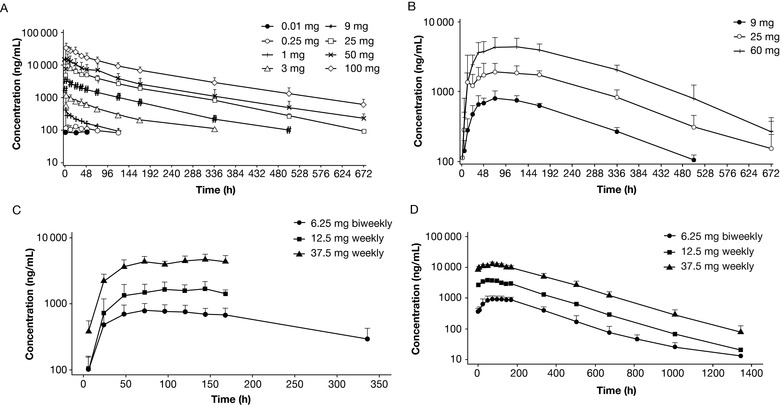
We report pharmacokinetics, pharmacodynamics, and safety of a novel anti‐CD28 domain antibody antagonist (lulizumab pegol) in healthy subjects following single‐ or multiple‐dose administration. A minimal anticipated biological effect level approach was used to select a 0.01 mg starting dose for a single‐ascending‐dose (SAD), double‐blind, first‐in‐human study. Part 1 included 9 intravenous (IV; 0.01‐100 mg) and 3 subcutaneous (SC; 9‐50 mg) doses or placebo. In part 2, a keyhole limpet hemocyanin (KLH) immunization was performed in 16 subjects/panel, who received 1 of 3 IV doses (9‐100 mg) or placebo. In a double‐blind, multiple‐ascending‐dose (MAD) study, subjects received SC lulizumab 6.25 mg every 2 weeks, 12.5 mg weekly, 37.5 mg weekly, or placebo. Among 180 treated subjects, 169 completed the studies. Peak concentrations and areas under the curve from time 0 to infinity increased dose proportionally. Estimated SC bioavailability was 68.2%. Receptor occupancy of approximately ≥80% was maintained for ≥2 weeks at ≥9‐mg doses (SAD) and throughout the dosing interval (MAD). IV doses ≥9 mg inhibited antibody production against KLH for 2 weeks. No significant cytokine or immune cell changes were observed. No immunogenicity responses persisted, and there was no correlation to adverse events. Headache occurred in 21 SAD and 4 MAD subjects receiving lulizumab; in the MAD study 5 lulizumab subjects experienced infections. Lulizumab IV or SC was safe at all doses studied, without evidence of cytokine release.
一种新型抗cd28结构域抗体拮抗剂在健康人体内的药代动力学、药效学和安全性研究
我们报告了一种新型抗cd28结构域抗体拮抗剂(lulizumab pegol)在健康受试者单次或多次给药后的药代动力学、药效学和安全性。采用最小预期生物效应水平方法选择0.01 mg起始剂量进行单次上升剂量(SAD)、双盲、首次人体研究。第一部分包括9例静脉注射(IV;0.01-100 mg)和3次皮下注射(SC;9-50毫克)或安慰剂。在第二部分中,对16名受试者/小组进行了锁孔帽贝血青素(KLH)免疫接种,他们接受了3次静脉注射剂量(9-100 mg)中的1次或安慰剂。在一项双盲、多重递增剂量(MAD)研究中,受试者接受每2周6.25 mg、12.5 mg /周、37.5 mg /周或安慰剂的单抗注射。在180名受试者中,169人完成了研究。从时间0到无穷远,峰值浓度和曲线下面积成比例地增加剂量。估计SC的生物利用度为68.2%。在≥9 mg剂量(SAD)和整个给药间隔(MAD)下,受体占用率约≥80%维持≥2周。IV剂量≥9mg抑制KLH抗体产生2周。未观察到明显的细胞因子或免疫细胞变化。没有免疫原性反应持续存在,也没有与不良事件相关。接受鲁利珠单抗治疗的21例SAD和4例MAD患者出现头痛;在MAD研究中,5名鲁里珠单抗受试者出现感染。Lulizumab IV或SC在所有研究剂量下都是安全的,没有细胞因子释放的证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.10
自引率
3.40%
发文量
176
审稿时长
2 months
期刊介绍:
The Journal of Clinical Pharmacology (JCP) is a Human Pharmacology journal designed to provide physicians, pharmacists, research scientists, regulatory scientists, drug developers and academic colleagues a forum to present research in all aspects of Clinical Pharmacology. This includes original research in pharmacokinetics, pharmacogenetics/pharmacogenomics, pharmacometrics, physiologic based pharmacokinetic modeling, drug interactions, therapeutic drug monitoring, regulatory sciences (including unique methods of data analysis), special population studies, drug development, pharmacovigilance, womens’ health, pediatric pharmacology, and pharmacodynamics. Additionally, JCP publishes review articles, commentaries and educational manuscripts. The Journal also serves as an instrument to disseminate Public Policy statements from the American College of Clinical Pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


