The broken “Off” switch in cancer signaling: PP2A as a regulator of tumorigenesis, drug resistance, and immune surveillance
Abstract
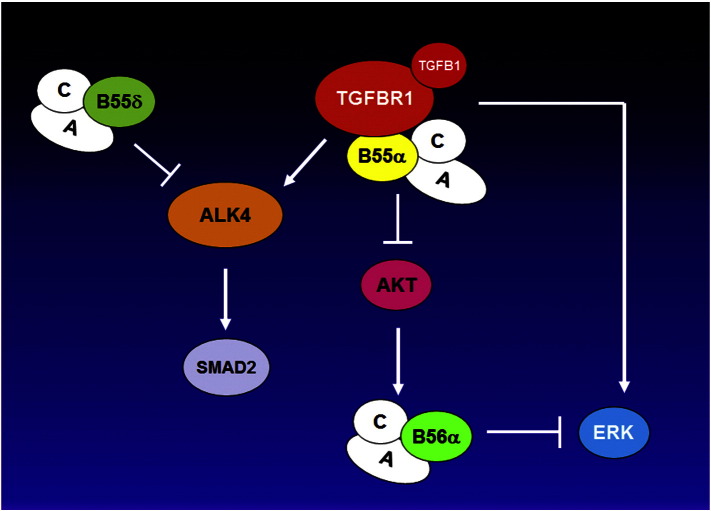
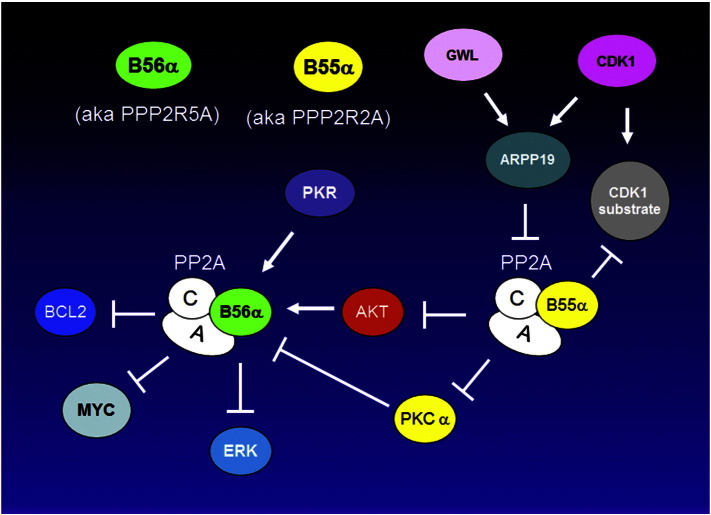
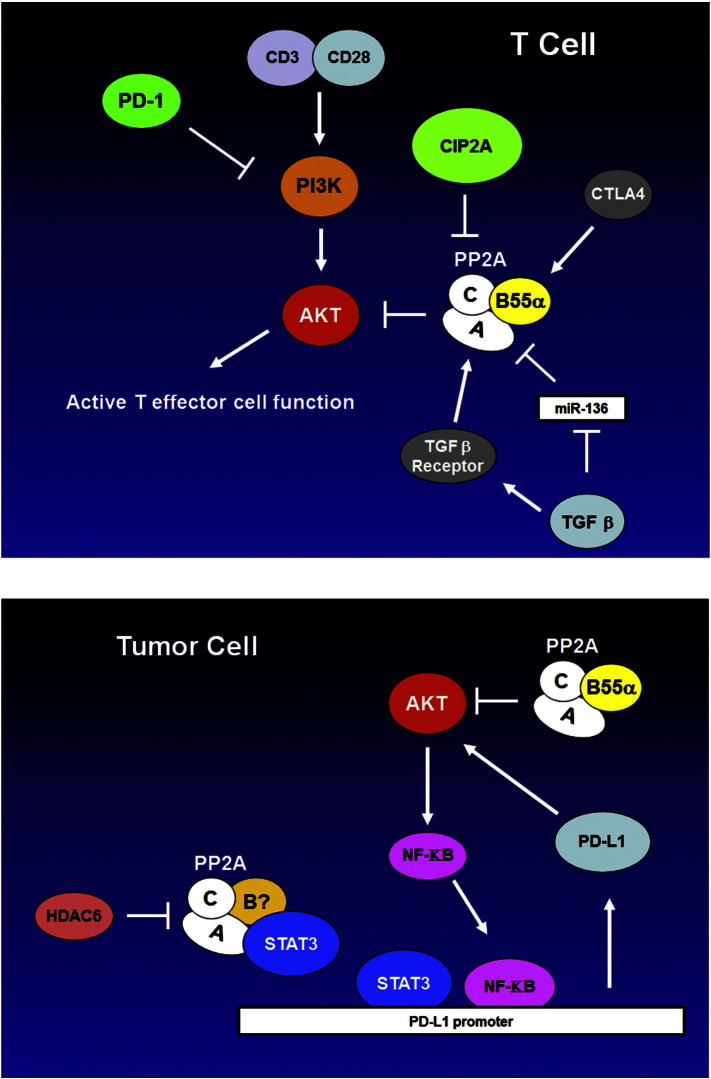
Aberrant activation of signal transduction pathways can transform a normal cell to a malignant one and can impart survival properties that render cancer cells resistant to therapy. A diverse set of cascades have been implicated in various cancers including those mediated by serine/threonine kinases such RAS, PI3K/AKT, and PKC. Signal transduction is a dynamic process involving both “On” and “Off” switches. Activating mutations of RAS or PI3K can be viewed as the switch being stuck in the “On” position resulting in continued signaling by a survival and/or proliferation pathway. On the other hand, inactivation of protein phosphatases such as the PP2A family can be seen as the defective “Off” switch that similarly can activate these pathways. A problem for therapeutic targeting of PP2A is that the enzyme is a hetero-trimer and thus drug targeting involves complex structures. More importantly, since PP2A isoforms generally act as tumor suppressors one would want to activate these enzymes rather than suppress them. The elucidation of the role of cellular inhibitors like SET and CIP2A in cancer suggests that targeting these proteins can have therapeutic efficacy by mechanisms involving PP2A activation. Furthermore, drugs such as FTY-720 can activate PP2A isoforms directly. This review will cover the current state of knowledge of PP2A role as a tumor suppressor in cancer cells and as a mediator of processes that can impact drug resistance and immune surveillance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


