Allogeneic hematopoietic cell transplantation can overcome the adverse prognosis indicated by secondary-type mutations in de novo acute myeloid leukemia
IF 5.2
2区 医学
Q1 HEMATOLOGY
引用次数: 7
Abstract
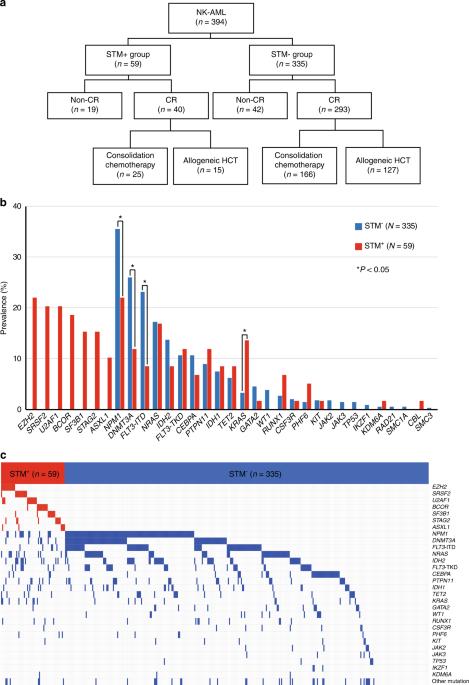
Secondary-type mutations (STMs), namely SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, and STAG2, are more frequently detected in secondary acute myeloid leukemia (AML) than in de novo AML. Whether de novo AML with STMs should be differently managed is, however, unclear. In 394 patients diagnosed with de novo AML who had a normal karyotype, the genetic profiling via targeted deep sequencing of 45 genes revealed 59 patients carrying STMs (STM+). The STM+ group showed shorter overall survival (OS) than the STM− group (5-year OS, 15.3 vs. 31.0%) (hazard ratio [HR]: 1.975, 95% confidence interval [CI]: 1.446–2.699, p < 0.001). Among the 40 STM+ patients who achieved CR, those who received allogeneic HCT (n = 15) showed better OS (5-year OS, 40.0 vs. 12.0%) (HR: 0.423, 95% CI: 0.184–0.975, p = 0.043) and relapse-free survival (5-year, 40.0 vs. 8.0%) (HR: 0.438, 95% CI: 0.189–1.015, p = 0.054) than those who received consolidation chemotherapy only. The cumulative incidence of relapse was lower in the patients who received allogeneic HCT (5-year, 33.3 vs. 60.0%) (HR: 0.288, 95% CI: 0.111–0.746, p = 0.011), and non-relapse mortality was similar between the two groups (p = 0.935). In conclusion, STM is an independent prognostic factor for adverse outcomes in AML that can be overcome by allogeneic HCT.
异基因造血细胞移植可克服新生急性髓性白血病二次型突变所显示的不良预后
继发性急性髓细胞白血病(AML)比新生急性髓细胞白血病更常检测到二次型突变(STMs),即 SRSF2、SF3B1、U2AF1、ZRSR2、ASXL1、EZH2、BCOR 和 STAG2。然而,是否应该对带有 STMs 的新发急性髓细胞白血病进行不同的处理,目前尚不清楚。在394例染色体核型正常的新发急性髓细胞白血病患者中,通过对45个基因进行靶向深度测序的基因谱分析发现,有59例患者携带STMs(STM+)。STM+组的总生存期(OS)短于STM-组(5年OS,15.3% vs. 31.0%)(危险比 [HR]:1.975,95% 置信区间 [CI]:1.446-2.699,P< 0.001)。在获得 CR 的 40 名 STM+ 患者中,接受异基因 HCT 的患者(n = 15)的 OS(5 年 OS,40.0 vs. 12.0%)(HR:0.423,95% CI:0.184-0.975,p = 0.043)和无复发生存期(5 年,40.0 vs. 8.0%)(HR:0.438,95% CI:0.189-1.015,p = 0.054)均优于仅接受巩固化疗的患者。接受异基因造血干细胞移植的患者的累计复发率较低(5 年,33.3% 对 60.0%)(HR:0.288,95% CI:0.111-0.746,p = 0.011),两组患者的非复发死亡率相似(p = 0.935)。总之,STM是导致急性髓细胞性白血病不良预后的一个独立因素,异基因造血干细胞移植可以克服这一因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bone Marrow Transplantation
医学-免疫学
CiteScore
8.40
自引率
8.30%
发文量
337
审稿时长
6 months
期刊介绍:
Bone Marrow Transplantation publishes high quality, peer reviewed original research that addresses all aspects of basic biology and clinical use of haemopoietic stem cell transplantation.
The broad scope of the journal thus encompasses topics such as stem cell biology, e.g., kinetics and cytokine control, transplantation immunology e.g., HLA and matching techniques, translational research, and clinical results of specific transplant protocols. Bone Marrow Transplantation publishes 24 issues a year.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


