The RAG Model: A New Paradigm for Genetic Risk Stratification in Multiple Myeloma.
引用次数: 6
Abstract
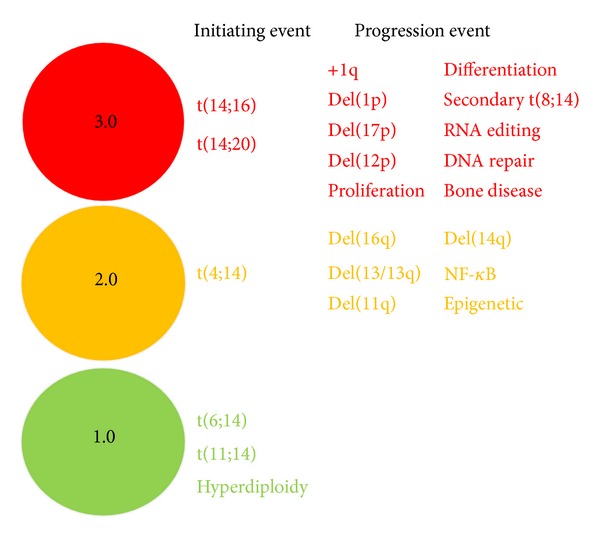
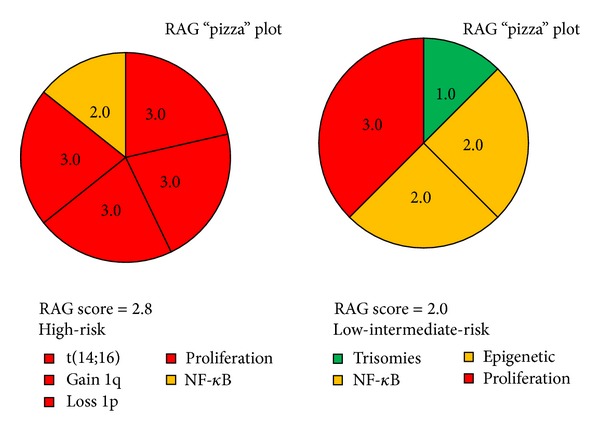
Molecular studies have shown that multiple myeloma is a highly genetically heterogonous disease which may manifest itself as any number of diverse subtypes each with variable clinicopathological features and outcomes. Given this genetic heterogeneity, a universal approach to treatment of myeloma is unlikely to be successful for all patients and instead we should strive for the goal of personalised therapy using rationally informed targeted strategies. Current DNA sequencing technologies allow for whole genome and exome analysis of patient myeloma samples that yield vast amounts of genetic data and provide a mutational overview of the disease. However, the clinical utility of this information currently lags far behind the sequencing technology which is increasingly being incorporated into clinical practice. This paper attempts to address this shortcoming by proposing a novel genetically based “traffic-light” risk stratification system for myeloma, termed the RAG (Red, Amber, Green) model, which represents a simplified concept of how complex genetic data may be compressed into an aggregate risk score. The model aims to incorporate all known clinically important trisomies, translocations, and mutations in myeloma and utilise these to produce a score between 1.0 and 3.0 that can be incorporated into diagnostic, prognostic, and treatment algorithms for the patient.
RAG模型:多发性骨髓瘤遗传风险分层的新范式。
分子研究表明,多发性骨髓瘤是一种高度遗传异质性的疾病,可能表现为任意数量的不同亚型,每种亚型具有不同的临床病理特征和结果。鉴于这种遗传异质性,治疗骨髓瘤的通用方法不太可能对所有患者都成功,相反,我们应该努力实现个性化治疗的目标,使用合理的有针对性的策略。目前的DNA测序技术允许对骨髓瘤患者样本进行全基因组和外显子组分析,从而产生大量的遗传数据,并提供疾病的突变概况。然而,这些信息的临床应用目前远远落后于越来越多地纳入临床实践的测序技术。本文试图通过提出一种新的基于遗传的骨髓瘤“红绿灯”风险分层系统来解决这一缺点,称为RAG (Red, Amber, Green)模型,它代表了如何将复杂的遗传数据压缩成总体风险评分的简化概念。该模型旨在纳入骨髓瘤中所有已知的临床上重要的三体、易位和突变,并利用这些来产生1.0到3.0之间的评分,该评分可以纳入患者的诊断、预后和治疗算法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


