Synthesis and Biological Evaluation of Macrocyclized Betulin Derivatives as a Novel Class of Anti-HIV-1 Maturation Inhibitors.
Q2 Pharmacology, Toxicology and Pharmaceutics
Open Medicinal Chemistry Journal
Pub Date : 2014-09-03
eCollection Date: 2014-01-01
DOI:10.2174/1874104501408010023
引用次数: 20
Abstract
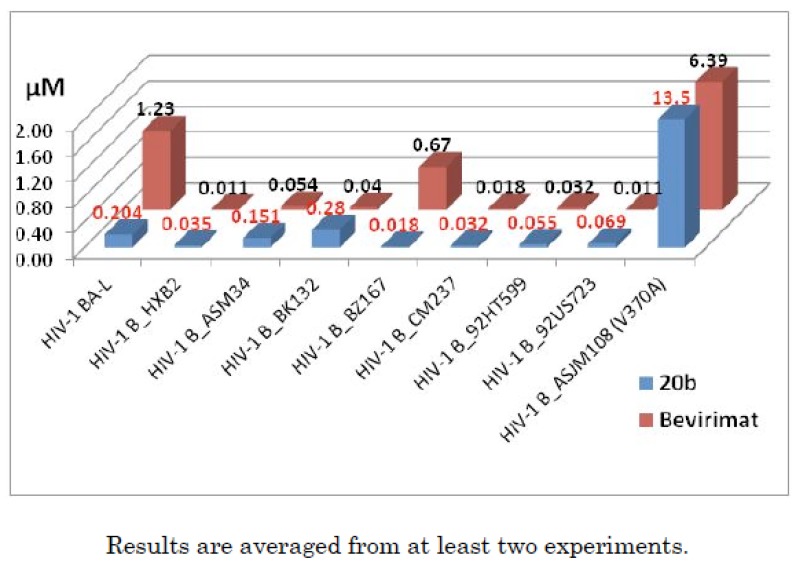
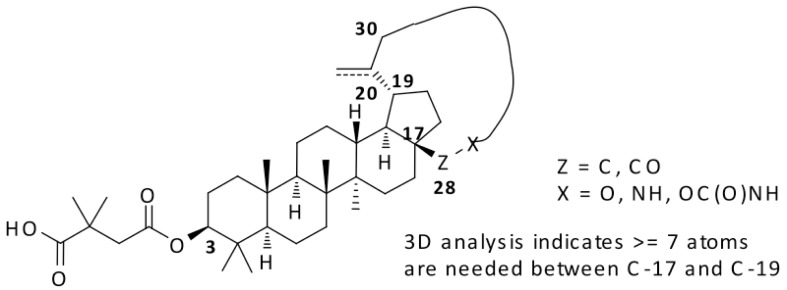
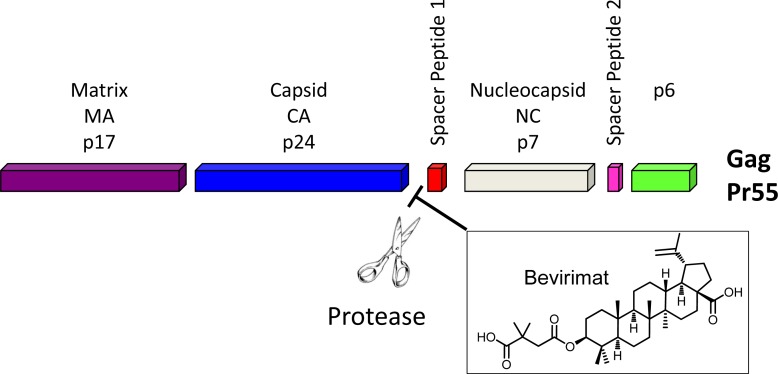
A macrocycle provides diverse functionality and stereochemical complexity in a conformationally preorganized ring structure, and it occupies a unique chemical space in drug discovery. However, the synthetic challenge to access this structural class is high and hinders the exploration of macrocycles. In this study, efficient synthetic routes to macrocyclized betulin derivatives have been established. The macrocycle containing compounds showed equal potency compared to bevirimat in multiple HIV-1 antiviral assays. The synthesis and biological evaluation of this novel series of HIV-1 maturation inhibitors will be discussed.
一类新型抗hiv -1成熟抑制剂大环化桦木素衍生物的合成及生物学评价
巨环在构象预组织的环结构中具有多种功能和立体化学的复杂性,在药物发现中占有独特的化学空间。然而,获得这种结构类的合成挑战很高,阻碍了对宏观环的探索。本研究建立了高效的大环化白桦脂衍生物的合成路线。在多次HIV-1抗病毒试验中,含有大环的化合物与bevirimat表现出相同的效力。本文将讨论这一系列新型HIV-1成熟抑制剂的合成和生物学评价。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Open Medicinal Chemistry Journal
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
4.40
自引率
0.00%
发文量
4
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


