Allergenic characterization of new mutant forms of Pru p 3 as new immunotherapy vaccines.
Clinical & Developmental Immunology
Pub Date : 2013-01-01
Epub Date: 2013-11-14
DOI:10.1155/2013/385615
引用次数: 7
Abstract
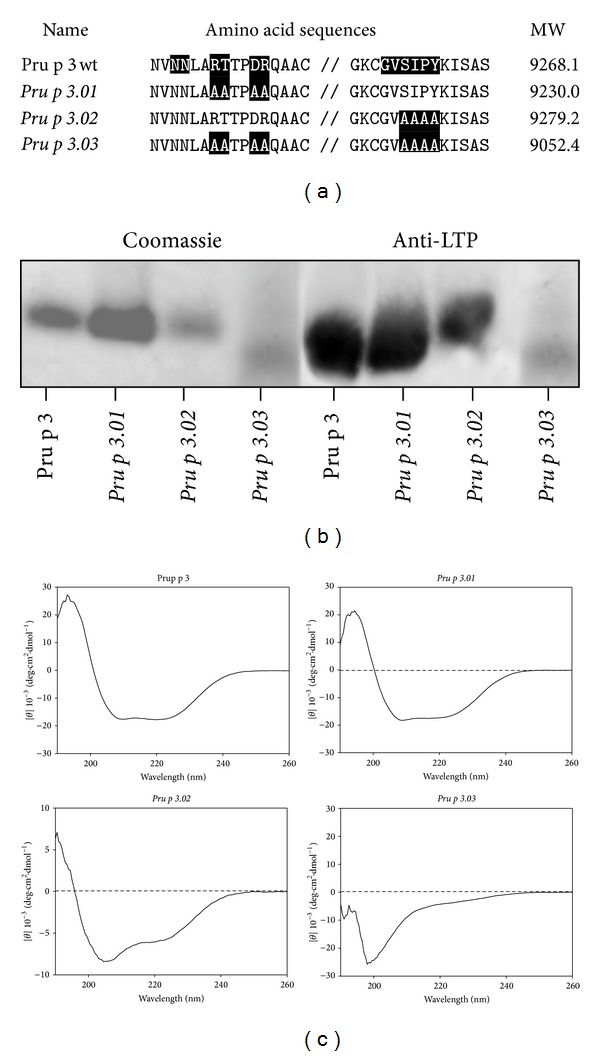
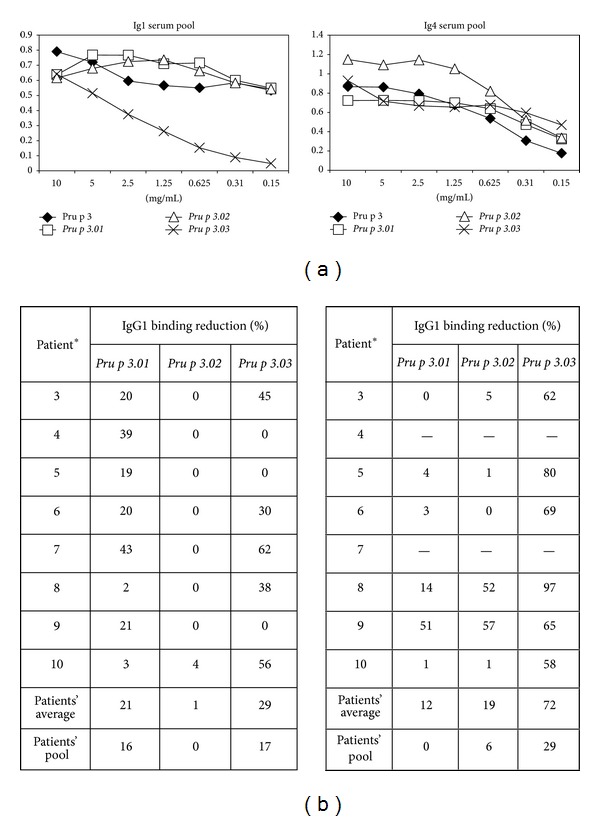
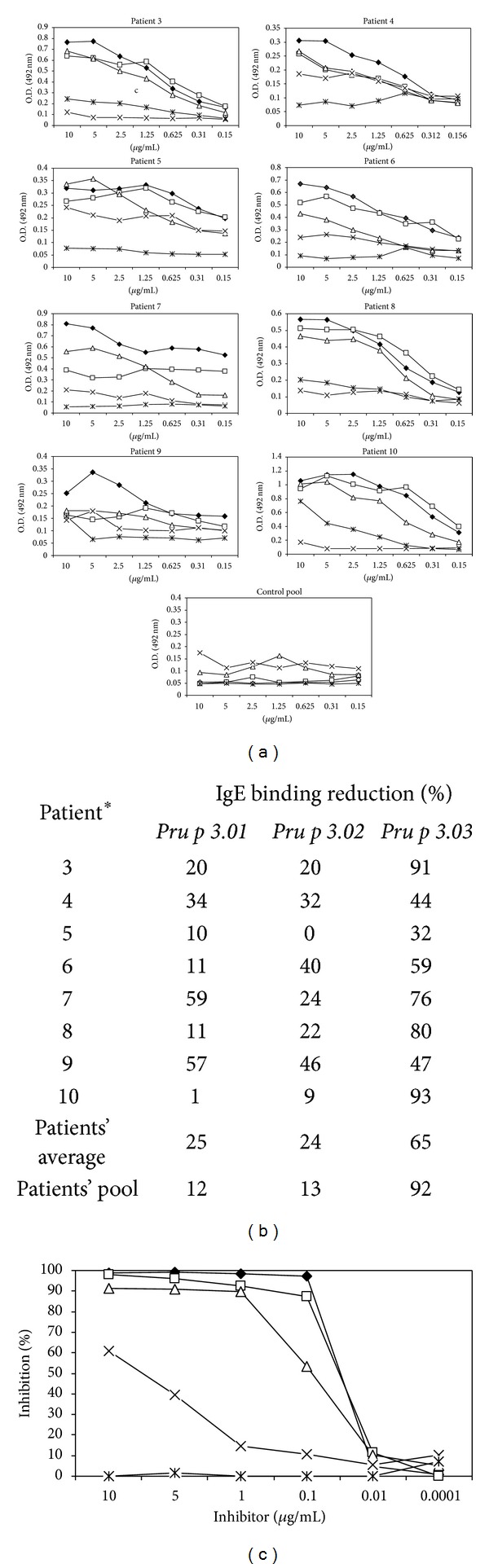
Nowadays, treatment of food allergy only considered the avoidance of the specific food. However, the possibility of cross-reactivity makes this practice not very effective. Immunotherapy may exhibit as a good alternative to food allergy treatment. The use of hypoallergenic molecules with reduced IgE binding capacity but with ability to stimulate the immune system is a promising tool which could be developed for immunotherapy. In this study, three mutants of Pru p 3, the principal allergen of peach, were produced based on the described mimotope and T cell epitopes, by changing the specific residues to alanine, named as Pru p 3.01, Pru p 3.02, and Pru p 3.03. Pru p 3.01 showed very similar allergenic activity as the wild type by in vitro assays. However, Pru p 3.02 and Pru p 3.03 presented reduced IgE binding with respect to the native form, by in vitro, ex vivo, and in vivo assays. In addition, Pru p 3.03 had affected the IgG4 binding capacity and presented a random circular dichroism, which was reflected in the nonrecognition by specific antibodies anti-Pru p 3. Nevertheless, both Pru p 3.02 and Pru p 3.03 maintained the binding to IgG1 and their ability to activate T lymphocytes. Thus, Pru p 3.02 and Pru p 3.03 could be good candidates for potential immunotherapy in peach-allergic patients.
Pru p3作为新免疫疗法疫苗的新突变形式的致敏特性。
如今,食物过敏的治疗只考虑避免食用特定的食物。然而,交叉反应的可能性使得这种做法不是很有效。免疫疗法可能是一种很好的替代食物过敏治疗的方法。使用IgE结合能力降低但能够刺激免疫系统的低致敏分子是一种很有前途的免疫治疗工具。在本研究中,通过将特异性残基改为丙氨酸,在所述模拟表位和T细胞表位的基础上产生了桃主要过敏原Pru p3的三个突变体,分别命名为Pru p3.01、Pru p3.02和Pru p3.03。Pru p 3.01通过体外测定显示出与野生型非常相似的致敏活性。然而,通过体外、离体和体内测定,相对于天然形式,Pru p 3.02和Pru p 3.0 3表现出减少的IgE结合。此外,Pru p 3.03影响了IgG4的结合能力,并呈现出随机的圆二色性,这反映在抗Pru p 3的特异性抗体的不识别上。尽管如此,Pru p 3.02和Pru p 3.0 3都保持了与IgG1的结合及其激活T淋巴细胞的能力。因此,Pru p 3.02和Pru p 3.0 3可能是桃过敏患者潜在免疫疗法的良好候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


