Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress
IF 8.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 169
Abstract
Sirtuin 3 (Sirt3), a major mitochondrial NAD+-dependent deacetylase, targets various mitochondrial proteins for lysine deacetylation and regulates important cellular functions such as energy metabolism, aging, and stress response. In this study, we identified the human 8-oxoguanine-DNA glycosylase 1 (OGG1), a DNA repair enzyme that excises 7,8-dihydro-8-oxoguanine (8-oxoG) from damaged genome, as a new target protein for Sirt3. We found that Sirt3 physically associated with OGG1 and deacetylated this DNA glycosylase and that deacetylation by Sirt3 prevented the degradation of the OGG1 protein and controlled its incision activity. We further showed that regulation of the acetylation and turnover of OGG1 by Sirt3 played a critical role in repairing mitochondrial DNA (mtDNA) damage, protecting mitochondrial integrity, and preventing apoptotic cell death under oxidative stress. We observed that following ionizing radiation, human tumor cells with silencing of Sirt3 expression exhibited deteriorated oxidative damage of mtDNA, as measured by the accumulation of 8-oxoG and 4977 common deletion, and showed more severe mitochondrial dysfunction and underwent greater apoptosis in comparison with the cells without silencing of Sirt3 expression. The results reported here not only reveal a new function and mechanism for Sirt3 in defending the mitochondrial genome against oxidative damage and protecting from the genotoxic stress-induced apoptotic cell death but also provide evidence supporting a new mtDNA repair pathway.
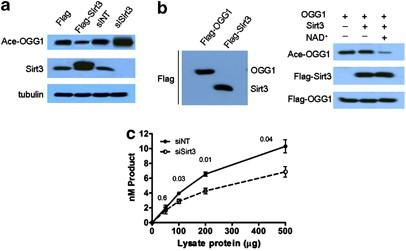
Sirt3 与 OGG1 的相互作用有助于线粒体 DNA 的修复,并在氧化应激下保护细胞免于凋亡
Sirtuin 3(Sirt3)是一种主要的线粒体 NAD+依赖性去乙酰化酶,它以各种线粒体蛋白为赖氨酸去乙酰化靶标,并调节能量代谢、衰老和应激反应等重要的细胞功能。在这项研究中,我们发现人类 8-氧鸟嘌呤-DNA糖基化酶 1(OGG1)是 Sirt3 的一个新靶蛋白,它是一种 DNA 修复酶,能从受损基因组中切除 7,8-二氢-8-氧鸟嘌呤(8-oxoG)。我们发现,Sirt3 与 OGG1 发生了物理关联,并对这种 DNA 糖基化酶进行了脱乙酰化,Sirt3 的脱乙酰化作用阻止了 OGG1 蛋白的降解,并控制了其切割活性。我们进一步发现,Sirt3 对 OGG1 的乙酰化和周转的调控在修复线粒体 DNA(mtDNA)损伤、保护线粒体完整性以及防止细胞在氧化应激下凋亡方面发挥了关键作用。我们观察到,与未沉默 Sirt3 表达的细胞相比,沉默 Sirt3 表达的人类肿瘤细胞在电离辐射后表现出更严重的 mtDNA 氧化损伤(以 8-oxoG 的积累和 4977 共缺失来衡量)、更严重的线粒体功能障碍和更严重的细胞凋亡。本文报告的结果不仅揭示了 Sirt3 在保护线粒体基因组免受氧化损伤和防止基因毒性应激诱导的细胞凋亡方面的新功能和新机制,而且还提供了支持新的 mtDNA 修复途径的证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death & Disease
CELL BIOLOGY-
CiteScore
15.10
自引率
2.20%
发文量
935
审稿时长
2 months
期刊介绍:
Brought to readers by the editorial team of Cell Death & Differentiation, Cell Death & Disease is an online peer-reviewed journal specializing in translational cell death research. It covers a wide range of topics in experimental and internal medicine, including cancer, immunity, neuroscience, and now cancer metabolism.
Cell Death & Disease seeks to encompass the breadth of translational implications of cell death, and topics of particular concentration will include, but are not limited to, the following:
Experimental medicine
Cancer
Immunity
Internal medicine
Neuroscience
Cancer metabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


