Photogenerated electrophilic radicals for the umpolung of enolate chemistry
IF 12.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology C: Photochemistry Reviews
Pub Date : 2021-03-01
DOI:10.1016/j.jphotochemrev.2020.100387
引用次数: 5
Abstract
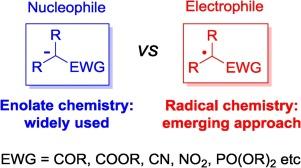
The use of enolate chemistry is the election choice when a CC bond formation is required exploiting the acidity of carbonyl derivatives in the α position. However, a reversed-polarity equivalent of enolate chemistry is emerging making use of electrophilic radicals having a radical site in place of a negative charge in the same α position. Visible light photoredox catalysis is becoming the ideal tool for the generation of these radicals thus allowing their wide application in several synthetic routes. Aim of this review is to collect recent examples of the chemistry of photogenerated electrophilic radicals for the forging of new C
C or other C
Y bonds.
烯酸酯化学中光生亲电自由基的研究
当需要利用α位置羰基衍生物的酸性形成CC键时,使用烯醇酯化学是一种选择。然而,一种相反极性的烯醇酸化学正在出现,利用亲电自由基在相同的α位置上具有一个自由基位点代替负电荷。可见光光氧化还原催化正在成为生成这些自由基的理想工具,从而使它们在几种合成路线中得到广泛应用。本综述的目的是收集最近的例子,光产生的亲电自由基的化学锻造新的CC或其他CY键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.90
自引率
0.70%
发文量
36
审稿时长
47 days
期刊介绍:
The Journal of Photochemistry and Photobiology C: Photochemistry Reviews, published by Elsevier, is the official journal of the Japanese Photochemistry Association. It serves as a platform for scientists across various fields of photochemistry to communicate and collaborate, aiming to foster new interdisciplinary research areas. The journal covers a wide scope, including fundamental molecular photochemistry, organic and inorganic photochemistry, photoelectrochemistry, photocatalysis, solar energy conversion, photobiology, and more. It provides a forum for discussing advancements and promoting collaboration in the field of photochemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


