Zhengjia Chen, Zhibo Wang, Haibin Wang, Taofeek K Owonikoko, Jeanne Kowalski, Fadlo R Khuri
{"title":"Interactive Software \"Isotonic Design using Normalized Equivalent Toxicity Score (ID-NETS©TM)\" for Cancer Phase I Clinical Trials.","authors":"Zhengjia Chen, Zhibo Wang, Haibin Wang, Taofeek K Owonikoko, Jeanne Kowalski, Fadlo R Khuri","doi":"10.2174/1874431101307010008","DOIUrl":null,"url":null,"abstract":"<p><p>Isotonic Design using Normalized Equivalent Toxicity Score (ID-NETS) is a novel Phase I design that integrates the novel toxicity scoring system originally proposed by Chen et al. [1] and the original Isotonic Design proposed by Leung et al. [2]. ID-NETS has substantially improved the accuracy of maximum tolerated dose (MTD) estimation and trial efficiency in the Phase I clinical trial setting by fully utilizing all toxicities experienced by each patient and treating toxicity response as a quasi-continuous variable instead of a binary indicator of dose limiting toxicity (DLT). To facilitate the incorporation of the ID-NETS method into the design and conduct of Phase I clinical trials, we have designed and developed a user-friendly software, ID-NETS(©TM), which has two functions: 1) Calculating the recommended dose for the subsequent patient cohort using available completed data; and 2) Performing simulations to obtain the operating characteristics of a trial designed with ID-NETS. Currently, ID-NETS(©TM)v1.0 is available for free download at http://winshipbbisr.emory.edu/IDNETS.html. </p>","PeriodicalId":88331,"journal":{"name":"The open medical informatics journal","volume":"7 ","pages":"8-17"},"PeriodicalIF":0.0000,"publicationDate":"2013-04-05","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://ftp.ncbi.nlm.nih.gov/pub/pmc/oa_pdf/12/17/TOMINFOJ-7-8.PMC3680993.pdf","citationCount":"3","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The open medical informatics journal","FirstCategoryId":"1085","ListUrlMain":"https://doi.org/10.2174/1874431101307010008","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"2013/1/1 0:00:00","PubModel":"Print","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 3
Abstract
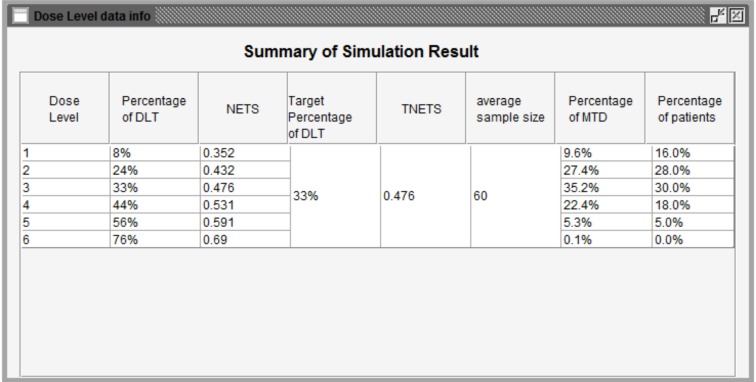
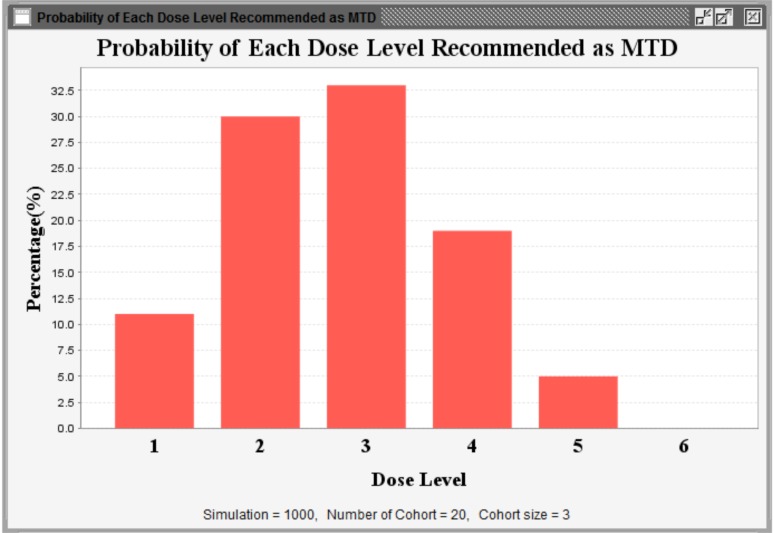
Isotonic Design using Normalized Equivalent Toxicity Score (ID-NETS) is a novel Phase I design that integrates the novel toxicity scoring system originally proposed by Chen et al. [1] and the original Isotonic Design proposed by Leung et al. [2]. ID-NETS has substantially improved the accuracy of maximum tolerated dose (MTD) estimation and trial efficiency in the Phase I clinical trial setting by fully utilizing all toxicities experienced by each patient and treating toxicity response as a quasi-continuous variable instead of a binary indicator of dose limiting toxicity (DLT). To facilitate the incorporation of the ID-NETS method into the design and conduct of Phase I clinical trials, we have designed and developed a user-friendly software, ID-NETS(©TM), which has two functions: 1) Calculating the recommended dose for the subsequent patient cohort using available completed data; and 2) Performing simulations to obtain the operating characteristics of a trial designed with ID-NETS. Currently, ID-NETS(©TM)v1.0 is available for free download at http://winshipbbisr.emory.edu/IDNETS.html.
用于癌症I期临床试验的交互式软件“使用标准化等效毒性评分(ID-NETS©TM)的等渗设计”。
采用归一化等效毒性评分(Normalized Equivalent Toxicity Score, ID-NETS)的等渗设计是将Chen等人[1]提出的新型毒性评分系统与Leung等人[2]提出的等渗设计相结合的一种新型I期设计。ID-NETS通过充分利用每位患者所经历的所有毒性,并将毒性反应作为准连续变量而不是剂量限制毒性(DLT)的二元指标,大大提高了I期临床试验中最大耐受剂量(MTD)估计的准确性和试验效率。为了便于将ID-NETS方法纳入I期临床试验的设计和实施,我们设计并开发了一个用户友好的软件ID-NETS(©TM),该软件具有两个功能:1)使用现有的完整数据计算后续患者队列的推荐剂量;2)进行仿真,获得使用ID-NETS设计的试验的工作特性。目前,ID-NETS(©TM)v1.0可在http://winshipbbisr.emory.edu/IDNETS.html免费下载。
本文章由计算机程序翻译,如有差异,请以英文原文为准。




 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


