Biochemical insights into the function of phage G1 gp67 in Staphylococcus aureus.
引用次数: 6
Abstract
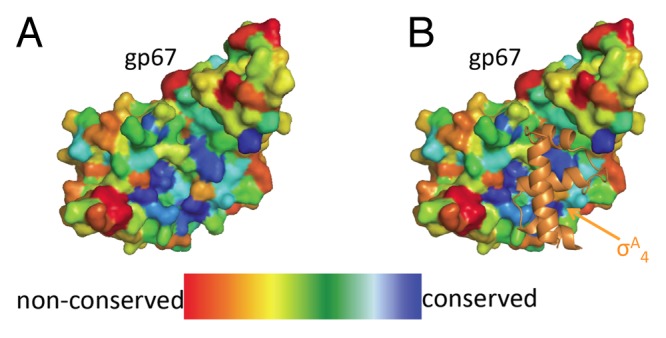
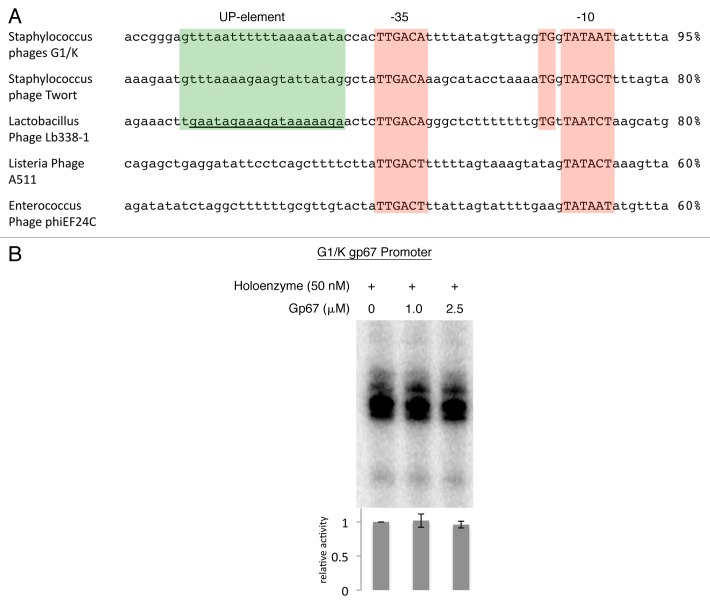
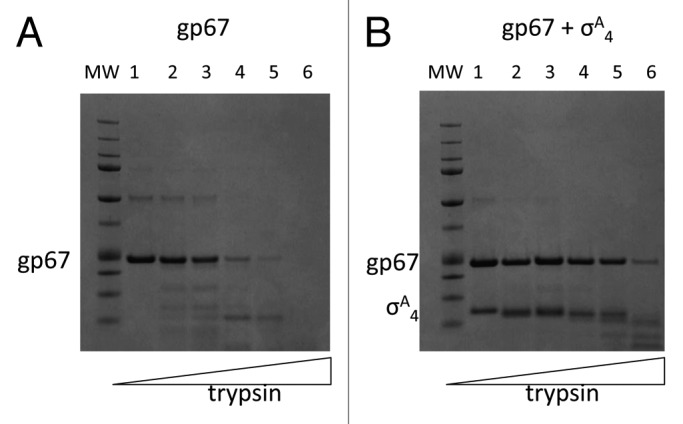
Bacteriophage (phage) are among the most diverse and abundant life forms on Earth. Studies have recently used phage diversity to identify novel antimicrobial peptides and proteins. We showed that one such phage protein, Staphylococcus aureus (Sau) phage G1 gp67, inhibits cell growth in Sau by an unusual mechanism. Gp67 binds to the host RNA polymerase (RNAP) through an interaction with the promoter specificity σ subunit, but unlike many other σ-binding phage proteins, gp67 does not disrupt transcription at most promoters. Rather, gp67 prevents binding of another RNAP domain, the α-C-terminal domain, to upstream A/T-rich elements required for robust transcription at rRNA promoters. Here, we discuss additional biochemical insights on gp67, how phage promoters escape the inhibitory function of gp67, and methodological advancements that were foundational to our work.
金黄色葡萄球菌噬菌体G1 gp67功能的生化分析。
噬菌体(噬菌体)是地球上最多样化和丰富的生命形式之一。最近的研究利用噬菌体多样性来鉴定新的抗菌肽和蛋白质。我们发现一种这样的噬菌体蛋白,金黄色葡萄球菌(Sau)噬菌体G1 gp67,通过一种不寻常的机制抑制Sau中的细胞生长。Gp67通过与启动子特异性σ亚基的相互作用与宿主RNA聚合酶(RNAP)结合,但与许多其他σ结合噬菌体蛋白不同,Gp67不会破坏大多数启动子的转录。相反,gp67阻止另一个RNAP结构域α- c末端结构域与上游富含A/ t的元件的结合,这是rRNA启动子转录所需的。在这里,我们讨论了gp67的其他生化见解,噬菌体启动子如何逃避gp67的抑制功能,以及为我们的工作奠定基础的方法进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


